| Weight | 247.247 g/mol |
|---|---|
| Formula | C10H17NO6 |
| Hydrogen Acceptors | 7 |
| Hydrogen Donors | 4 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 3 |
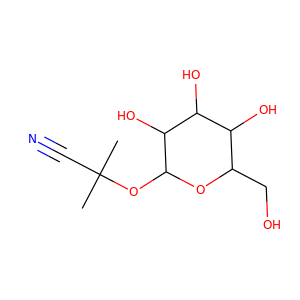
LINAMARIN (554-35-8)
 Score:
#1306 in Biochemistry
,
#3199 in Biology
,
#4001 in Chemistry
Score:
#1306 in Biochemistry
,
#3199 in Biology
,
#4001 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin: cloning, functional expression in Pichia pastoris, and substrate specificity of the isolated recombinant enzymes. (Journal of Biological Chemistry, 2000)
- Studies on cassava, Manihot utilissima PohlI. Cyanogenesis: The biosynthesis of linamarin and lotaustralin in etiolated seedlings (Phytochemistry, 1968)
- Biosynthesis of the Nitrile Glucosides Rhodiocyanoside A and D and the Cyanogenic Glucosides Lotaustralin and Linamarin in Lotus japonicus (Plant Physiology, 2004)
- The distribution of the cyanoglucosides linamarin and lotaustralin in higher plants (Phytochemistry, 1965)
- Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire (Tropical Medicine & International Health, 1997)
- OCCURRENCE, VARIATION AND BIOSYNTHESIS OF THE CYANOGENIC GLUCOSIDES LINAMARIN AND LOTAUSTRALIN IN SPECIES OF THE HELICONIINI (INSECTA: LEPIDOPTERA) (Comparative Biochemistry and Physiology B, 1983)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC(C)(C#N)OC1C(C(C(C(O1)CO)O)O)O
-
InChIKeyQLTCHMYAEJEXBT-UHFFFAOYSA-N
- Pubchem - LINAMARIN
- Wikipedia - linamarin
Linamarin is a cyanogenic glucoside found in the leaves and roots of plants such as cassava, lima beans, and flax. It is a glucoside of acetone cyanohydrin. Upon exposure to enzymes and gut flora in the human intestine, linamarin and its methylated relative lotaustralin can decompose to the toxic chemical hydrogen cyanide; hence food uses of plants that contain significant quantities of linamarin require extensive preparation and detoxification.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
1,5-Anhydro-D-glucitol
(154-58-5)
1 alternate name
3 suppliers
trans-4-tert-Butylcyclohexanecarboxylic acid
(5451-55-8, 943-28-2)
1 alternate name
3 suppliers
2-tert-Butyl-4-methoxyphenol
(121-00-6, 25013-16-5)
1 alternate name
3 suppliers
Glufosfamide
(132682-98-5)
1 alternate name
3 suppliers
4-tert-Butylbenzoic acid
(98-73-7)
1 alternate name
3 safety hazards
3 suppliers
2,6-DI-TERT-BUTYLPHENOL
(128-39-2)
1 alternate name
3 suppliers
2-acetamido-2-deoxy-beta-D-glucopyranosylamine
(14131-68-1)
1 alternate name
3 suppliers
1,6-Anhydro-beta-D-glucopyranose
(498-07-7)
1 alternate name
3 suppliers
phytosphingosine
(554-62-1)
1 alternate name
3 suppliers
Leucoglucosan
(498-07-7)
1 alternate name
3 suppliers
5,6-Dihydro-5-azacytidine
(62402-31-7, 62488-57-7)
1 alternate name
3 suppliers
3025-88-5
(3025-88-5)
1 alternate name
3 suppliers
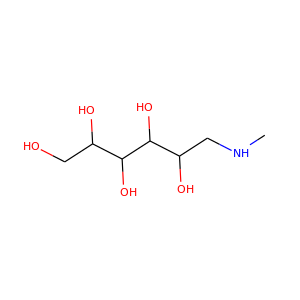
N-Methyl-D-glucamine
(6284-40-8)
1 alternate name
3 suppliers
d-iduronic acid
(3402-98-0)
1 alternate name
3 suppliers
meglumine
(6284-40-8)
1 alternate name
3 suppliers
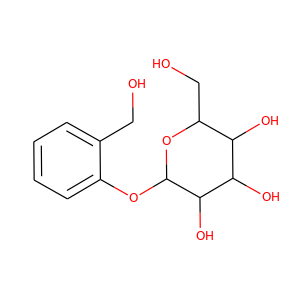
salicin
(138-52-3)
1 alternate name
3 suppliers
Croscarmellose
(9000-11-7)
1 alternate name
3 suppliers
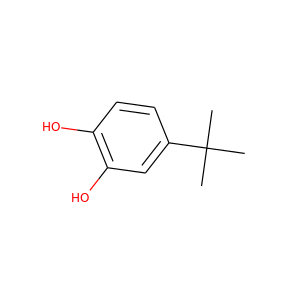
4-tert-Butylcatechol
(98-29-3, 27213-78-1)
1 alternate name
3 suppliers
CAS Directory


 LINAMARIN
LINAMARIN
