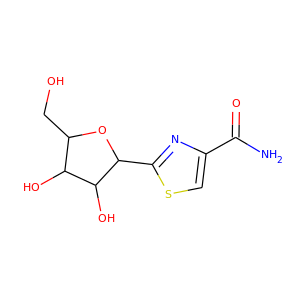
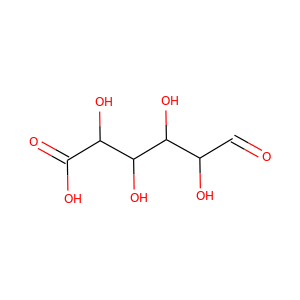
| Weight | 260.271 g/mol |
|---|---|
| Formula | C9H12N2O5S |
| Hydrogen Acceptors | 7 |
| Hydrogen Donors | 4 |
| Aromatic Rings | 1 |
| Rotatable Bonds | 3 |
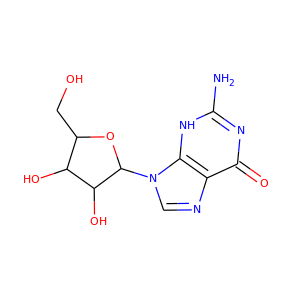
TIAZOFURIN (60084-10-8)
 Score:
#1789 in Biochemistry
,
#3911 in Biology
,
#4924 in Chemistry
Score:
#1789 in Biochemistry
,
#3911 in Biology
,
#4924 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for TIAZOFURIN
- Fisher Scientific - Search for TIAZOFURIN
- TCI - Search for TIAZOFURIN
- Biochemically Directed Therapy of Leukemia with Tiazofurin, a Selective Blocker of Inosine 5-Phosphate Dehydrogenase Activity (Cancer Research, 1989)
- Modulation of IMP dehydrogenase activity and guanylate metabolism by tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide). (Journal of Biological Chemistry, 1984)
- Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses. (Antimicrobial Agents and Chemotherapy, 1984)
- Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. (Cancer Research, 1985)
- Furanfurin and Thiophenfurin: Two Novel Tiazofurin Analogues. Synthesis, Structure, Antitumor Activity, and Interactions with Inosine Monophosphate Dehydrogenase (Journal of Medicinal Chemistry, 1995)
- Ribavirin, tiazofurin, and selenazofurin: mononucleotides and nicotinamide adenine dinucleotide analogues. Synthesis, structure, and interactions with IMP dehydrogenase (Journal of Medicinal Chemistry, 1985)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESC1=C(N=C(S1)C2C(C(C(O2)CO)O)O)C(=O)N
-
InChIKeyFVRDYQYEVDDKCR-UHFFFAOYSA-N
- Pubchem - TIAZOFURIN
- Wikipedia - tiazofurin
Tiazofurin is an inhibitor of IMP dehydrogenase. Tiazofurin and its analogues are under investigation for potential use in the treatment of cancer.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
nicotinamide riboside
(1341-23-7)
1 alternate name
3 suppliers
5,6-Dihydro-5-azacytidine
(62402-31-7, 62488-57-7)
1 alternate name
3 suppliers
Aminoimidazole ribotide
(25635-88-5)
1 alternate name
3 suppliers
guanosine
(118-00-3)
1 alternate name
3 suppliers
xanthosine
(5968-90-1, 146-80-5)
1 alternate name
3 suppliers
endothall
(28874-46-6, 145-73-3)
1 alternate name
5 safety hazards
3 suppliers
UNII-18F7018HG1
(28871-63-8)
1 alternate name
3 suppliers
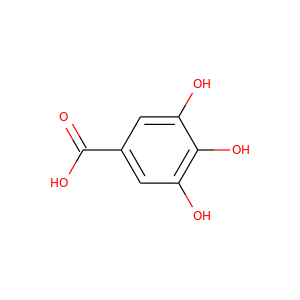
Gallic acid
(149-91-7)
1 alternate name
3 suppliers
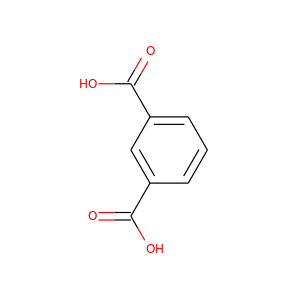
ISOPHTHALIC ACID
(121-91-5)
1 alternate name
3 suppliers
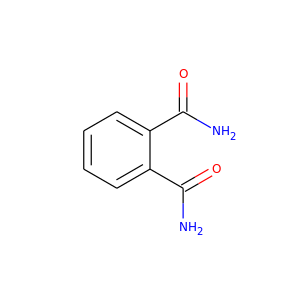
PHTHALAMIDE
(88-96-0)
1 alternate name
3 suppliers
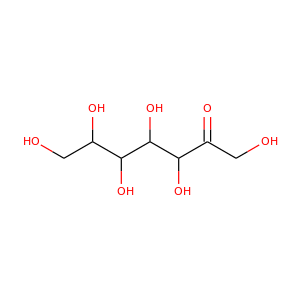
D-manno-Heptulose
(29325-35-7, 3019-74-7, 3615-44-9)
1 alternate name
3 suppliers
3,4-Dihydroxymandelic acid
(14883-87-5, 775-01-9)
1 alternate name
3 suppliers
ANCITABINE
(31698-14-3, 10212-25-6)
1 alternate name
3 suppliers
CYTARABINE HYDROCHLORIDE
(69-74-9)
1 alternate name
3 suppliers
CYTARABINE HYDROCHLORIDE
(69-74-9)
1 alternate name
3 suppliers
2,4,5-trichlorophenoxyacetic acid
(35915-18-5, 93-76-5)
1 alternate name
7 safety hazards
3 suppliers
ganciclovir
(82410-32-0)
1 alternate name
3 suppliers
TEREPHTHALIC ACID
(100-21-0)
1 alternate name
2 safety hazards
3 suppliers
1,5-Anhydro-D-glucitol
(154-58-5)
1 alternate name
3 suppliers
Cytidine-3'-Monophosphate
(84-52-6)
1 alternate name
3 suppliers
d-iduronic acid
(3402-98-0)
1 alternate name
3 suppliers
2-Naphthoxyacetic acid
(120-23-0)
1 alternate name
3 suppliers
CAS Directory