| Weight | 450.619 g/mol |
|---|---|
| Formula | C29H38O4 |
| Hydrogen Acceptors | 3 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 1 |
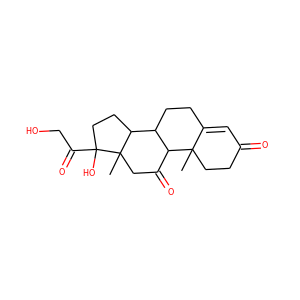
celastrol (34157-83-0)
 Score:
#1649 in Biochemistry
,
#3726 in Biology
,
#4690 in Chemistry
Score:
#1649 in Biochemistry
,
#3726 in Biology
,
#4690 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for celastrol
- Fisher Scientific - Search for celastrol
- TCI - celastrol270.00 USD
- Celastrol, a Triterpene Extracted from the Chinese Thunder of God Vine, Is a Potent Proteasome Inhibitor and Suppresses Human Prostate Cancer Growth in Nude Mice (Cancer Research, 2006)
- Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease (Progress in Neuro-psychopharmacology & Biological Psychiatry, 2001)
- Celastrol, a Novel Triterpene, Potentiates TNF-Induced Apoptosis and Suppresses Invasion of Tumor Cells by Inhibiting NF-B-Regulated Gene Products and TAK1-Mediated NF-B Activation (Blood, 2006)
- Inhibition of NF-B activation through targeting IB kinase by celastrol, a quinone methide triterpenoid (Biochemical Pharmacology, 2006)
- Celastrol: Molecular targets of Thunder God Vine. (Biochemical and Biophysical Research Communications, 2010)
- Activation of Heat Shock and Antioxidant Responses by the Natural Product Celastrol: Transcriptional Signatures of a Thiol-targeted Molecule (Molecular Biology of the Cell, 2007)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC1=C(C(=O)C=C2C1=CC=C3C2(CCC4(C3(CCC5(C4CC(CC5)(C)C(=O)O)C)C)C)C)O
-
InChIKeyKQJSQWZMSAGSHN-UHFFFAOYSA-N
- Pubchem - celastrol
- Wikipedia - celastrol
Celastrol (tripterine) is a chemical compound isolated from the root extracts of Tripterygium wilfordii (Thunder god vine) and Celastrus regelii. Celastrol is a pentacyclic triterpenoid and belongs to the family of quinone methides. In in vitro and in vivo animal experiments, celastrol exhibits antioxidant, anti-inflammatory, anticancer, and insecticidal activities.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
hydrocortisone hemisuccinate
(2203-97-6, 125-04-2)
1 alternate name
3 suppliers
Cortexolone
(152-58-9)
1 alternate name
3 suppliers
BETA-TOCOPHEROL
(148-03-8, 16698-35-4)
1 alternate name
3 suppliers
PYRETHRIN II
(121-29-9)
1 alternate name
4 safety hazards
3 suppliers
STIGMASTEROL
(83-48-7)
1 alternate name
3 suppliers
Tocopherols
(7616-22-0, 1406-66-2)
1 alternate name
3 suppliers
Spirosol-5-en-3-ol
(126-17-0)
1 alternate name
3 suppliers
solasodine
(126-17-0)
1 alternate name
3 suppliers
Buccalsone
(125-04-2)
1 alternate name
3 suppliers
18-HYDROXYCORTICOSTERONE
(561-65-9)
1 alternate name
3 suppliers
96-69-5
(96-69-5, 57062-46-1)
1 alternate name
1 safety hazard
3 suppliers
cortisone
(53-06-5)
1 alternate name
3 suppliers
BETA-SITOSTEROL
(68555-08-8, 19044-06-5, 83-46-5)
1 alternate name
3 suppliers
Dexamethasone 21-acetate
(987-24-6, 1177-87-3)
1 alternate name
3 suppliers
BETAMETHASONE ACETATE
(987-24-6)
1 alternate name
3 suppliers
UNII-1R89KK61YI
(73573-88-3, 84064-38-0)
1 alternate name
3 suppliers
carnosol
(5957-80-2)
1 alternate name
3 suppliers
Hydrocortisone 17-butyrate
(13609-67-1, 135467-84-4)
1 alternate name
3 suppliers
beclomethasone
(4419-39-0)
1 alternate name
3 suppliers
HYDROCORTISONE VALERATE
(57524-89-7)
1 alternate name
3 suppliers
Quinidine gluconate
(7054-25-3, 6587-33-3)
1 alternate name
3 suppliers
Quinidine gluconate
(7054-25-3)
1 alternate name
3 suppliers
4,4-Dimechol-8,14,24-trienol
(64284-64-6)
1 alternate name
3 suppliers
hydrocortisone butyrate
(135467-84-4)
1 alternate name
3 suppliers
PYRETHRIN I
(121-21-1)
1 alternate name
3 safety hazards
3 suppliers
Maslinic acid
(4373-41-5)
1 alternate name
3 suppliers
Ursolic acid
(77-52-1)
1 alternate name
3 suppliers
NSC167406
(77-52-1)
1 alternate name
3 suppliers
77-52-1
(77-52-1)
1 alternate name
3 suppliers
Prestwick_355
(77-52-1)
1 alternate name
3 suppliers
CAS Directory

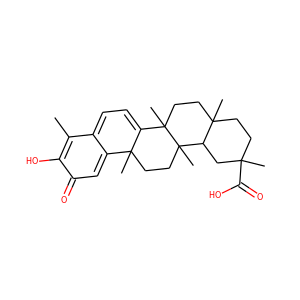
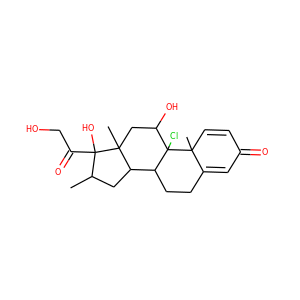
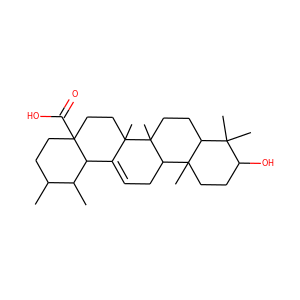

 celastrol
celastrol
