| Weight | 346.467 g/mol |
|---|---|
| Formula | C21H30O4 |
| Hydrogen Acceptors | 4 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 2 |
Cortexolone (152-58-9)
Reichstein's Substance S · 11 Desoxycortisol · Cortodoxone
 Score:
#480 in Microbiology
,
#1709 in Biochemistry
,
#3801 in Biology
,
#4783 in Chemistry
Score:
#480 in Microbiology
,
#1709 in Biochemistry
,
#3801 in Biology
,
#4783 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Cortexolone41.90 - 102.00 USD
- Fisher Scientific - Search for Cortexolone
- TCI - Cortexolone59.00 USD
- Glucocorticoid Receptors in Rat Thymocytes INTERACTIONS WITH THE ANTIGLUCOCORTICOID CORTEXOLONE AND MECHANISM OF ITS ACTION (Journal of Biological Chemistry, 1974)
- 11-Hydroxylation of Cortexolone (Reichstein Compound S) to Hydrocortisone by Curvularia lunata Entrapped in Photo-Cross-Linked Resin Gels (Applied and Environmental Microbiology, 1983)
- Cortexolone: Binding to glucocorticoid receptors in rat thymocytes and mechanism of its antiglucocorticoid action (Biochemical and Biophysical Research Communications, 1972)
- Reference levels for 17-hydroxyprogesterone, 11-desoxycortisol, cortisol, testosterone, dehydroepiandrosterone sulfate and androstenedione in infants from birth to six months of age. (European Journal of Pediatrics, 2008)
- Sequential conversion of cortexolone to prednisolone by immobilized mycelia of Curvularia lunata and immobilized cells of Arthrobacter simplex (Applied Microbiology and Biotechnology, 1985)
- THE INDEPENDENT ESTIMATION OF 11-DESOXYCORTISOL AND CORTISOL IN A SINGLE PLASMA SAMPLE* (The Journal of Clinical Endocrinology and Metabolism, 1961)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Reichstein's Substance S
- 11 Desoxycortisol
- Cortodoxone
- 11-Desoxycortisone
- 11 Desoxycortisone
- Reichsteins Substance S
- 11-Deoxycortisol
- Reichstein Substance S
- 11 Deoxycortisol
- 11-Desoxycortisol
-
SMILESCC12CCC(=O)C=C1CCC3C2CCC4(C3CCC4(C(=O)CO)O)C
-
InChIKeyWHBHBVVOGNECLV-UHFFFAOYSA-N
- Pubchem - Cortexolone
- Wikipedia - cortodoxone
11-Deoxycortisol, also known as cortodoxone (INN) or cortexolone, as well as 17,21-dihydroxyprogesterone or 17,21-dihydroxypregn-4-ene-3,20-dione, is a glucocorticoid steroid hormone. It was first synthesized by Tadeusz Reichstein, and has also been referred to as Reichstein's Substance. On April 5, 1952, biochemist Durey Peterson and microbiologist Herbert Murray at Upjohn published the first report of a breakthrough fermentation process for the microbial 11-oxygenation of steroids (e.g.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
cortisone
(53-06-5)
11 alternate names
3 suppliers
18-HYDROXYCORTICOSTERONE
(561-65-9)
11 alternate names
3 suppliers
Tetrahydrodeoxycorticosterone
(567-03-3, 567-02-2)
11 alternate names
3 suppliers
cycloheximide
(66-81-9)
11 alternate names
4 safety hazards
3 suppliers
ZEARALENONE
(17924-92-4, 36455-70-6)
11 alternate names
3 suppliers
estradiol valerate
(979-32-8)
11 alternate names
3 suppliers
hydrocortisone hemisuccinate
(2203-97-6, 125-04-2)
11 alternate names
3 suppliers
testosterone enanthate
(315-37-7)
11 alternate names
3 suppliers
Methoprene
(36557-27-4, 40596-69-8, 52020-07-2)
11 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
11 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
11 alternate names
3 suppliers
ERUCIC ACID
(1072-39-5, 112-86-7)
11 alternate names
3 suppliers
12-Hydroxystearic acid
(106-14-9, 36377-33-0, 18417-00-0, 27924-99-8, 5762-36-7)
11 alternate names
3 suppliers
NOOTKATONE
(4674-50-4, 28834-25-5)
11 alternate names
3 suppliers
54827-17-7
(54827-17-7)
11 alternate names
3 suppliers
4674-50-4
(91416-23-8, 4674-50-4)
11 alternate names
3 suppliers
UNII-ZMS1VJK5HY
(38427-78-0, 4674-50-4)
11 alternate names
3 suppliers
BENZILIC ACID
(76-93-7)
11 alternate names
1 safety hazard
3 suppliers
27-hydroxycholesterol
(20380-11-4)
11 alternate names
3 suppliers
CAS Directory

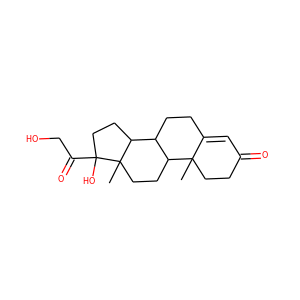
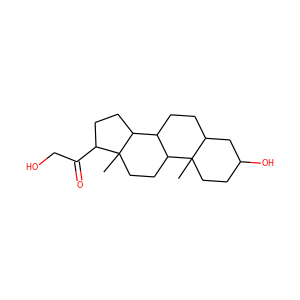
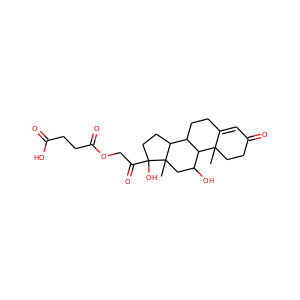

 Cortexolone
Cortexolone
