| Weight | 362.466 g/mol |
|---|---|
| Formula | C21H30O5 |
| Hydrogen Acceptors | 5 |
| Hydrogen Donors | 3 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 3 |
18-HYDROXYCORTICOSTERONE (561-65-9)
18-Hydrocorticosterone · 18 Hydrocorticosterone · 18 Hydroxycorticosterone
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - 18-HYDROXYCORTICOSTERONE195.00 - 739.00 USD
- Fisher Scientific - Search for 18-HYDROXYCORTICOSTERONE
- TCI - Search for 18-HYDROXYCORTICOSTERONE
- The Significance of Elevated Levels of Plasma 18-Hydroxycorticosterone in Patients with Primary Aldosteronism* (The Journal of Clinical Endocrinology and Metabolism, 1979)
- 18-Hydroxycorticosterone: A review (Journal of Steroid Biochemistry, 1978)
- Dose-Response Relationships Between Plasma Adrenocorticotropin (ACTH), Cortisol, Aldosterone, and 18-Hydroxycorticosterone After Injection of ACTH-(139) or Human Corticotropin-Releasing Hormone in Man* (The Journal of Clinical Endocrinology and Metabolism, 1988)
- Response of aldosterone and 18-hydroxycorticosterone to angiotensin II in normal subjects and patients with essential hypertension, Conn's syndrome, and nontumorous hyperaldosteronism. (Hypertension, 1981)
- Simultaneous Measurement of Secretory Rates of Aldosterone and 18-Hydroxycorticosterone (The Journal of Clinical Endocrinology and Metabolism, 1965)
- 18-Hydroxycorticosterone, 18-Hydroxycortisol, and 18-Oxocortisol in the Diagnosis of Primary Aldosteronism and Its Subtypes (The Journal of Clinical Endocrinology and Metabolism, 2012)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - 18-Hydrocorticosterone
- 18 Hydrocorticosterone
- 18 Hydroxycorticosterone
-
SMILESCC12CCC(=O)C=C1CCC3C2C(CC4(C3CCC4C(=O)CO)CO)O
-
InChIKeyHFSXHZZDNDGLQN-UHFFFAOYSA-N
- Pubchem - 18-HYDROXYCORTICOSTERONE
- Wikipedia - 18-hydroxycorticosterone
18-Hydroxycorticosterone is a derivative of corticosterone. It serves as an intermediate in the synthesis of aldosterone by the enzyme aldosterone synthase in the zona glomerulosa:
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
Cortexolone
(152-58-9)
4 alternate names
3 suppliers
cortisone
(53-06-5)
4 alternate names
3 suppliers
Tetrahydrodeoxycorticosterone
(567-03-3, 567-02-2)
4 alternate names
3 suppliers
hydrocortisone hemisuccinate
(2203-97-6, 125-04-2)
4 alternate names
3 suppliers
ZEARALENONE
(17924-92-4, 36455-70-6)
4 alternate names
3 suppliers
prostaglandin h2
(42935-17-1)
4 alternate names
3 suppliers
53-00-9
(53-00-9)
4 alternate names
3 suppliers
cycloheximide
(66-81-9)
4 alternate names
4 safety hazards
3 suppliers
testosterone enanthate
(315-37-7)
4 alternate names
3 suppliers
estradiol valerate
(979-32-8)
4 alternate names
3 suppliers
Prallethrin
(103065-19-6, 23031-36-9)
4 alternate names
3 safety hazards
3 suppliers
Prallethrin [ISO]
(23031-36-9)
4 alternate names
2 safety hazards
3 suppliers
NOOTKATONE
(4674-50-4, 28834-25-5)
4 alternate names
3 suppliers
4674-50-4
(91416-23-8, 4674-50-4)
4 alternate names
3 suppliers
UNII-ZMS1VJK5HY
(38427-78-0, 4674-50-4)
4 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
4 alternate names
3 suppliers
Methyl ricinoleate
(141-24-2)
4 alternate names
3 suppliers
methyl 12-hydroxyoctadec-9-enoate
(141-24-2)
4 alternate names
3 suppliers
ZERANOL
(26538-44-3, 55331-29-8)
4 alternate names
3 suppliers
prostaglandin I2
(61849-14-7, 35121-78-9)
4 alternate names
3 suppliers
HEMATOXYLIN
(517-28-2, 17647-60-8)
4 alternate names
3 suppliers
Haematoxylin
(517-28-2)
4 alternate names
3 suppliers
17-Hydroxypregnenolone
(387-79-1)
4 alternate names
3 suppliers
Glyceryl monostearate
(83138-62-9, 11099-07-3, 31566-31-1, 37349-34-1, 123-94-4, 22610-63-5, 14811-92-8, 85666-92-8)
4 alternate names
3 suppliers
HEMATOXYLIN
(517-28-2)
4 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
4 alternate names
3 suppliers
Methyl 12-hydroxyoctadecanoate
(141-23-1)
4 alternate names
3 suppliers
CAS Directory

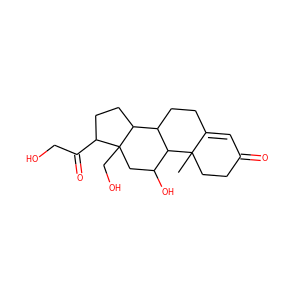
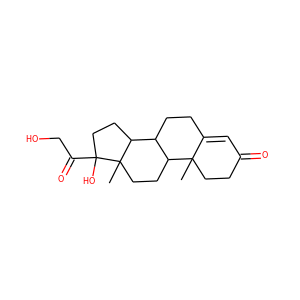
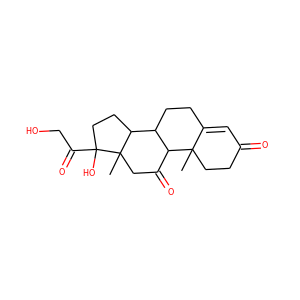
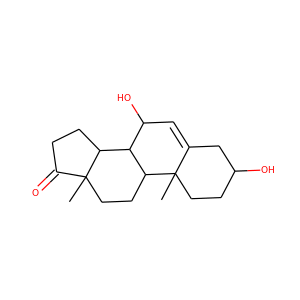

 18-HYDROXYCORTICOSTERONE
18-HYDROXYCORTICOSTERONE
