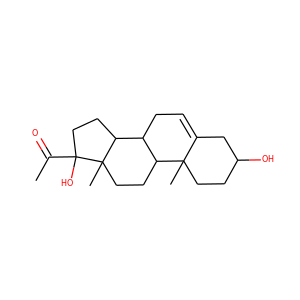
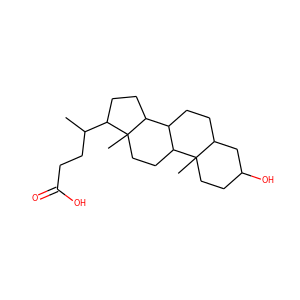
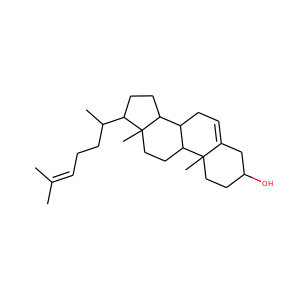
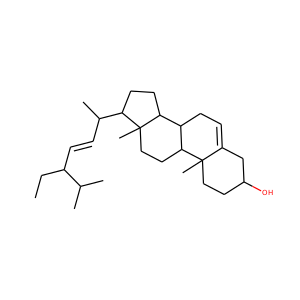
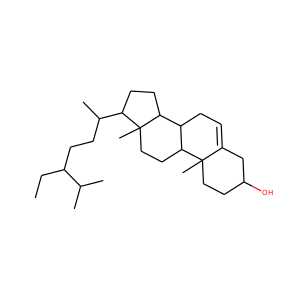
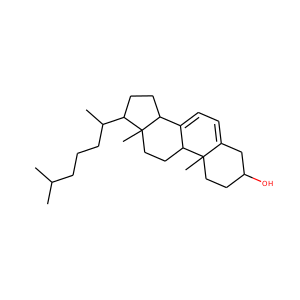
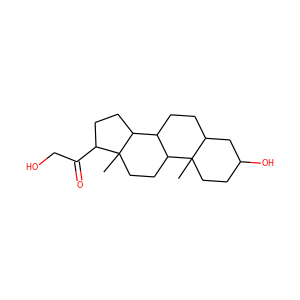
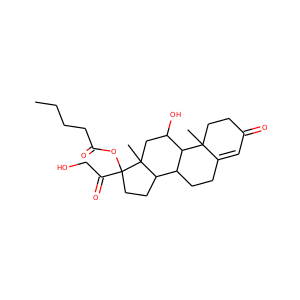
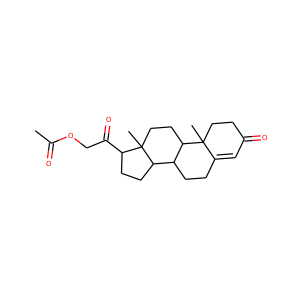
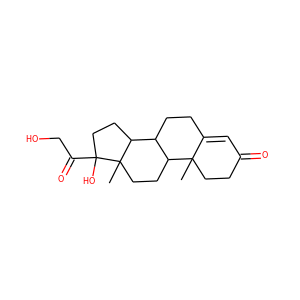
| Weight | 332.484 g/mol |
|---|---|
| Formula | C21H32O3 |
| Hydrogen Acceptors | 3 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 1 |
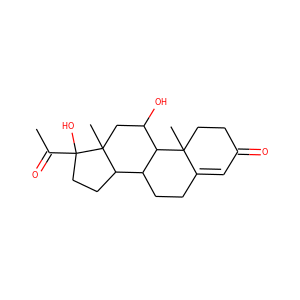
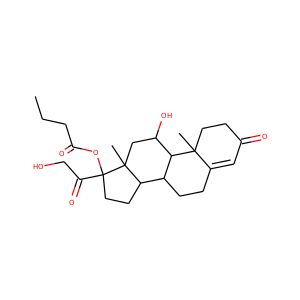
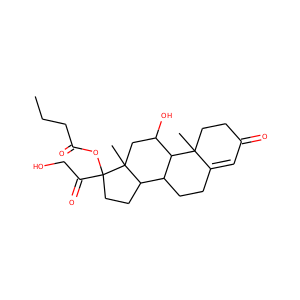
17-Hydroxypregnenolone (387-79-1)
Hydroxypregnenolone · 17-Hydroxypregnenolone, (3beta,13alpha,17alpha)-Isomer · 17-Hydroxypregnenolone, (3alpha)-Isomer
 Score:
#5 in Multidisciplinary scientific
,
#1564 in Biochemistry
,
#3599 in Biology
,
#4515 in Chemistry
Score:
#5 in Multidisciplinary scientific
,
#1564 in Biochemistry
,
#3599 in Biology
,
#4515 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for 17-Hydroxypregnenolone
- Fisher Scientific - Search for 17-Hydroxypregnenolone
- TCI - Search for 17-Hydroxypregnenolone
- Radioimmunoassay of Plasma Pregnenolone, 17-Hydroxypregnenolone and Dehydroepiandrosterone Under Various Physiological Conditions (The Journal of Clinical Endocrinology and Metabolism, 1973)
- 7-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system (Proceedings of the National Academy of Sciences of the United States of America, 2004)
- EVIDENCE THAT STEROID SULFATES SERVE AS BIOSYNTHETIC INTERMEDIATES. II. IN VITRO CONVERSION OF PREGNENOLONE-3H SULFATE-35S TO 17ALPHA-HYDROXYPREGNENOLONE-3H SULFATE-35S. (Biochemistry, 1964)
- 7-Hydroxypregnenolone Mediates Melatonin Action Underlying Diurnal Locomotor Rhythms (The Journal of Neuroscience, 2008)
- Simultaneous determination of 17-hydroxypregnenolone and 17-hydroxyprogesterone in dried blood spots from low birth weight infants using LCMS/MS (Journal of Pharmaceutical and Biomedical Analysis, 2008)
- Prolactin Increases the Synthesis of 7-Hydroxypregnenolone, a Key Factor for Induction of Locomotor Activity, in Breeding Male Newts (Endocrinology, 2010)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Hydroxypregnenolone
- 17-Hydroxypregnenolone, (3beta,13alpha,17alpha)-Isomer
- 17-Hydroxypregnenolone, (3alpha)-Isomer
- 17alpha-Hydroxypregnenolone
- 17 alpha Hydroxypregnenolone
- 17 alpha-Hydroxypregnenolone
- 17-Hydroxypregnenolone, (3beta,17alpha)-Isomer
- 17-Hydroxypregnenolone, (3beta,13alpha)-Isomer
- 17alpha Hydroxypregnenolone
- 17 Hydroxypregnenolone
- 17-alpha-Hydroxypregnenolone
-
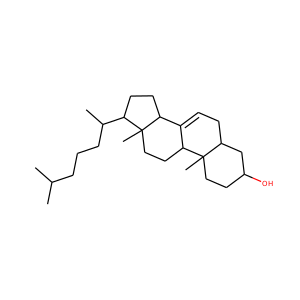
SMILESCC(=O)C1(CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)O
-
InChIKeyJERGUCIJOXJXHF-UHFFFAOYSA-N
- Pubchem - 17-Hydroxypregnenolone
- Wikipedia - 17-Hydroxypregnenolone
17-Hydroxypregnenolone is a pregnane (C21) steroid that is obtained by hydroxylation of pregnenolone at the C17 position. This step is performed by the mitochondrial cytochrome P450 enzyme 17-hydroxylase (CYP17A1) that is present in the adrenal and gonads. Peak levels are reached in humans at the end of puberty and then decline.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
27-hydroxycholesterol
(20380-11-4)
12 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
12 alternate names
3 suppliers
Cerebrosterol
(474-73-7)
12 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
12 alternate names
3 suppliers
22R-hydroxycholesterol
(22348-64-7, 17954-98-2)
12 alternate names
3 suppliers
hydroxyprogesterone
(604-09-1, 68-96-2)
12 alternate names
3 suppliers
17alpha-Hydroxypregn-4-ene-3,20-dione
(604-09-1)
12 alternate names
3 suppliers
DESMOSTEROL
(313-04-2)
12 alternate names
3 suppliers
STIGMASTEROL
(83-48-7)
12 alternate names
3 suppliers
BETA-SITOSTEROL
(68555-08-8, 19044-06-5, 83-46-5)
12 alternate names
3 suppliers
7-Dehydrocholesterol
(434-16-2)
12 alternate names
3 suppliers
Dihydrocholesterol
(80-97-7)
12 alternate names
3 suppliers
21-Deoxyhydrocortisone
(641-77-0)
12 alternate names
3 suppliers
Norethindrone acetate
(38673-38-0, 51-98-9)
12 alternate names
3 suppliers
19-Norethindrone acetate
(51-98-9)
12 alternate names
3 suppliers
Lathosterol
(80-99-9)
12 alternate names
3 suppliers
Zymosterol
(128-33-6)
12 alternate names
3 suppliers
Zymostenol
(566-97-2)
12 alternate names
3 suppliers
estradiol valerate
(979-32-8)
12 alternate names
3 suppliers
7-Ketodeoxycholic acid
(911-40-0)
12 alternate names
3 suppliers
20-beta-Progerol
(145-15-3, 145-14-2)
12 alternate names
3 suppliers
3,7-Dihydroxy-12-oxocholanoic acid
(2458-08-4)
12 alternate names
3 suppliers
Melengestro acetate
(425-51-4, 595-33-5)
12 alternate names
3 suppliers
Tetrahydrodeoxycorticosterone
(567-03-3, 567-02-2)
12 alternate names
3 suppliers
Glycolithocholic acid
(474-74-8)
12 alternate names
3 suppliers
53-00-9
(53-00-9)
12 alternate names
3 suppliers
chlormadinone acetate
(302-22-7)
12 alternate names
3 suppliers
Hydrocortisone 17-butyrate
(13609-67-1, 135467-84-4)
12 alternate names
3 suppliers
4,4-Dimechol-8,14,24-trienol
(64284-64-6)
12 alternate names
3 suppliers
hydrocortisone butyrate
(135467-84-4)
12 alternate names
3 suppliers
ISOPHYTOL
(505-32-8)
12 alternate names
2 safety hazards
3 suppliers
HYDROCORTISONE VALERATE
(57524-89-7)
12 alternate names
3 suppliers
Coprocholic acid
(547-98-8)
12 alternate names
3 suppliers
hydrocortisone acetate
(50-03-3)
12 alternate names
3 suppliers
testosterone enanthate
(315-37-7)
12 alternate names
3 suppliers
prostaglandin h2
(42935-17-1)
12 alternate names
3 suppliers
Glycocholic acid
(475-31-0)
12 alternate names
3 suppliers
phytosphingosine
(554-62-1)
12 alternate names
3 suppliers
Deoxycorticosterone acetate
(56-47-3)
12 alternate names
3 suppliers
Cortexolone
(152-58-9)
12 alternate names
3 suppliers
21-Hydroxypregnenolone
(1164-98-3)
12 alternate names
3 suppliers
18-HYDROXYCORTICOSTERONE
(561-65-9)
12 alternate names
3 suppliers
Methyl 12-hydroxyoctadecanoate
(141-23-1)
12 alternate names
3 suppliers
CAS Directory