| Weight | 384.648 g/mol |
|---|---|
| Formula | C27H44O |
| Hydrogen Acceptors | 1 |
| Hydrogen Donors | 1 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 4 |
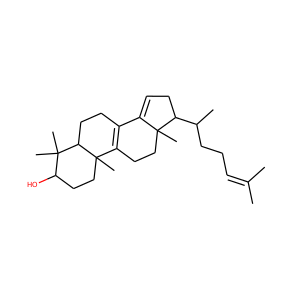
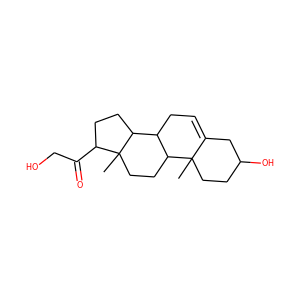
DESMOSTEROL (313-04-2)
24 Dehydrocholesterol · 24-Dehydrocholesterol · Demosterol
 Score:
#1491 in Biochemistry
,
#3499 in Biology
,
#4376 in Chemistry
Score:
#1491 in Biochemistry
,
#3499 in Biology
,
#4376 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - DESMOSTEROL62.90 - 108.00 USD
- Fisher Scientific - Search for DESMOSTEROL
- TCI - Search for DESMOSTEROL
- Regulated Accumulation of Desmosterol Integrates Macrophage Lipid Metabolism and Inflammatory Responses (Cell, 2012)
- Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. (Journal of Biological Chemistry, 1960)
- ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain (The FASEB Journal, 2007)
- Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. (Journal of Lipid Research, 1993)
- Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm (Journal of Lipid Research, 1998)
- Significance of Sterol Structural Specificity DESMOSTEROL CANNOT REPLACE CHOLESTEROL IN LIPID RAFTS (Journal of Biological Chemistry, 2006)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - 24 Dehydrocholesterol
- 24-Dehydrocholesterol
- Demosterol
-
SMILESCC(CCC=C(C)C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C
-
InChIKeyAVSXSVCZWQODGV-UHFFFAOYSA-N
- Pubchem - DESMOSTEROL
- Wikipedia - desmosterol
Desmosterol is a molecule similar to cholesterol. Desmosterol is the immediate precursor of cholesterol in the Bloch pathway of cholesterol biosynthesis. 24-dehydrocholesterol reductase catalyses the reduction of desmosterol to cholesterol.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
Zymosterol
(128-33-6)
4 alternate names
3 suppliers
7-Dehydrocholesterol
(434-16-2)
4 alternate names
3 suppliers
Dihydrocholesterol
(80-97-7)
4 alternate names
3 suppliers
22R-hydroxycholesterol
(22348-64-7, 17954-98-2)
4 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
4 alternate names
3 suppliers
27-hydroxycholesterol
(20380-11-4)
4 alternate names
3 suppliers
Cerebrosterol
(474-73-7)
4 alternate names
3 suppliers
Lathosterol
(80-99-9)
4 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
4 alternate names
3 suppliers
Zymostenol
(566-97-2)
4 alternate names
3 suppliers
4,4-Dimechol-8,14,24-trienol
(64284-64-6)
4 alternate names
3 suppliers
Glycolithocholic acid
(474-74-8)
4 alternate names
3 suppliers
STIGMASTEROL
(83-48-7)
4 alternate names
3 suppliers
BETA-SITOSTEROL
(68555-08-8, 19044-06-5, 83-46-5)
4 alternate names
3 suppliers
17-Hydroxypregnenolone
(387-79-1)
4 alternate names
3 suppliers
7-Ketodeoxycholic acid
(911-40-0)
4 alternate names
3 suppliers
3,7-Dihydroxy-12-oxocholanoic acid
(2458-08-4)
4 alternate names
3 suppliers
Coprocholic acid
(547-98-8)
4 alternate names
3 suppliers
estradiol valerate
(979-32-8)
4 alternate names
3 suppliers
Glycocholic acid
(475-31-0)
4 alternate names
3 suppliers
4-Cholesten-3-one
(601-57-0)
4 alternate names
3 suppliers
7alpha-Hydroxy-4-cholesten-3-one
(3862-25-7)
4 alternate names
3 suppliers
21-Hydroxypregnenolone
(1164-98-3)
4 alternate names
3 suppliers
testosterone enanthate
(315-37-7)
4 alternate names
3 suppliers
53-00-9
(53-00-9)
4 alternate names
3 suppliers
Norethindrone acetate
(38673-38-0, 51-98-9)
4 alternate names
3 suppliers
19-Norethindrone acetate
(51-98-9)
4 alternate names
3 suppliers
Testosterone undecanoate
(5949-44-0)
4 alternate names
3 suppliers
Deoxycorticosterone acetate
(56-47-3)
4 alternate names
3 suppliers
CAS Directory

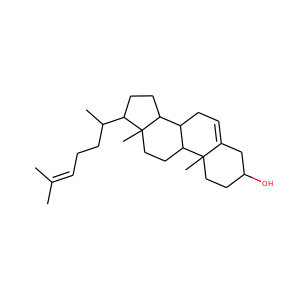
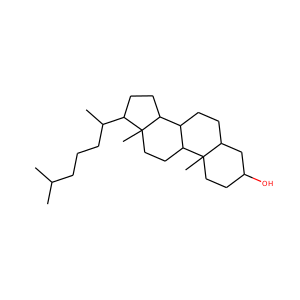
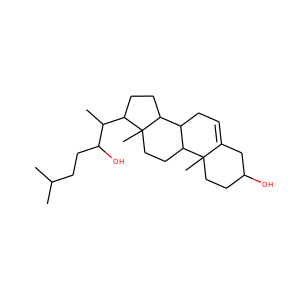
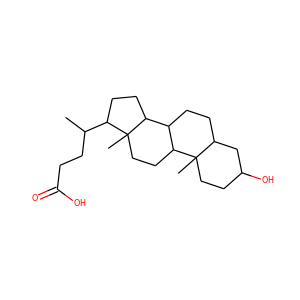
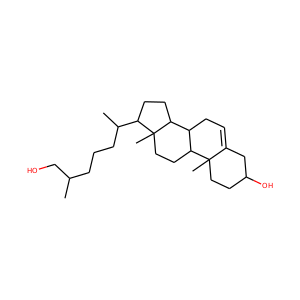
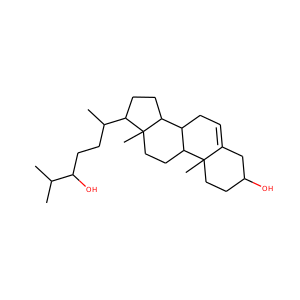
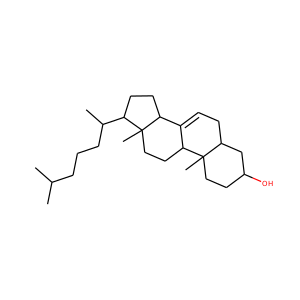
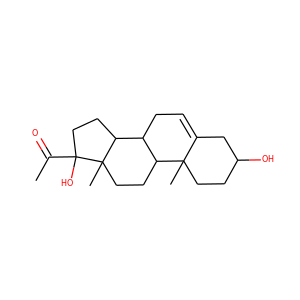

 DESMOSTEROL
DESMOSTEROL
