| Weight | 332.484 g/mol |
|---|---|
| Formula | C21H32O3 |
| Hydrogen Acceptors | 3 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 2 |
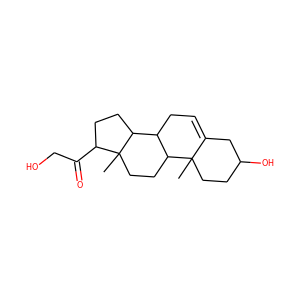
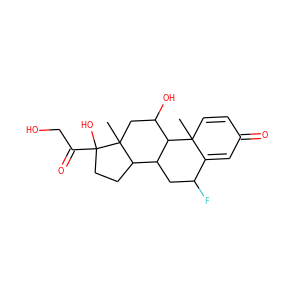
21-Hydroxypregnenolone (1164-98-3)
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for 21-Hydroxypregnenolone
- Fisher Scientific - Search for 21-Hydroxypregnenolone
- TCI - Search for 21-Hydroxypregnenolone
- Role of 21-hydroxypregnenolone and of 17-, 21-dihydroxypregnenolone in the biogenesis of C21 and C19 steroids by adrenal gland slices (Steroids, 1964)
- Role of 21-hydroxypregnenolone in the synthesis of corticosterone from pregnenolone by sheep adrenal tissue in vitro (General and Comparative Endocrinology, 1967)
- Steroid 21-hydroxylation by human preovulatory follicles from stimulated cycles: A mass spectrometrical study of deoxycorticosterone, 21-hydroxypregnenolone and 11-deoxycortisol in follicular fluid (Journal of Steroid Biochemistry, 1987)
- STUDIES ON THE METABOLISM OF C-21 STEROIDS IN THE HUMAN FOETO-PLACENTAL UNIT. I. FORMATION OF A BETA-UNSATURATED 3-KETONES IN MIDTERM PLACENTAS PERFUSED IN SITU WITH PREGNENOLONE AND 17-ALPHA-HYDROXYPREGNENOLONE. (European Journal of Endocrinology, 1965)
- Studies on the metabolism of corticosteroids in the human foeto-placental unit. 3. Role of 21-hydroxypregnenolone in the biosynthesis of corticosteroids. (European Journal of Endocrinology, 1970)
- A paradox: elevated 21-hydroxypregnenolone production in newborns with 21-hydroxylase deficiency (Steroids, 1987)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC12CCC3C(C1CCC2C(=O)CO)CC=C4C3(CCC(C4)O)C
-
InChIKeyMOIQRAOBRXUWGN-UHFFFAOYSA-N
- Pubchem - 21-Hydroxypregnenolone
- Wikipedia - 21-hydroxypregnenolone
21-Hydroxypregnenolone, also known as prebediolone, as well as 3,21-dihydroxypregn-5-en-20-one, is a naturally occurring, endogenous pregnane steroid and an intermediate in the biosynthesis of 11-deoxycorticosterone (21-hydroxyprogesterone), corticosterone (11,21-dihydroxyprogesterone), and other corticosteroids. It is formed from pregnenolone in the adrenal glands. The 21-acetate ester of 21-hydroxypregnenolone, prebediolone acetate, is described as a glucocorticoid and has been used in the treatment of rheumatoid arthritis.
Suppliers
Most-cited Publications
Safety
Alternate Names
External Links
Similar Compounds
53-00-9
(53-00-9)
1 alternate name
3 suppliers
desoxycorticosterone
(64-85-7)
1 alternate name
3 suppliers
16alpha-Hydroxyestrone
(566-76-7)
1 alternate name
3 suppliers
norethynodrel
(68-23-5)
1 alternate name
3 suppliers
stanozolol
(10418-03-8, 302-96-5)
1 alternate name
3 suppliers
2-HYDROXYESTRONE
(362-06-1)
1 alternate name
3 suppliers
302-97-6
(302-97-6)
1 alternate name
2 safety hazards
3 suppliers
2-METHOXYESTRONE
(362-08-3)
1 alternate name
3 suppliers
Methandienone
(72-63-9, 100-61-8)
1 alternate name
3 suppliers
7-Dehydrocholesterol
(434-16-2)
1 alternate name
3 suppliers
19-Hydroxyandrost-4-ene-3,17-dione
(510-64-5)
1 alternate name
3 suppliers
fluprednisolone
(53-34-9)
1 alternate name
3 suppliers
27-hydroxycholesterol
(20380-11-4)
1 alternate name
3 suppliers
Lathosterol
(80-99-9)
1 alternate name
3 suppliers
DESMOSTEROL
(313-04-2)
1 alternate name
3 suppliers
Dihydrocholesterol
(80-97-7)
1 alternate name
3 suppliers
Octadecanedioic acid
(871-70-5)
1 alternate name
3 suppliers
Zymostenol
(566-97-2)
1 alternate name
3 suppliers
TESTOSTERONE CYPIONATE
(58-20-8)
1 alternate name
3 suppliers
4376-20-9
(4376-20-9)
1 alternate name
3 suppliers
estradiol cypionate
(313-06-4)
1 alternate name
3 suppliers
Dodecyl gallate
(1166-52-5)
1 alternate name
1 safety hazard
3 suppliers
Zymosterol
(128-33-6)
1 alternate name
3 suppliers
Testosterone sulfate
(651-45-6)
1 alternate name
3 suppliers
22R-hydroxycholesterol
(22348-64-7, 17954-98-2)
1 alternate name
3 suppliers
Tanshinone IIA
(568-72-9)
1 alternate name
3 suppliers
CAS Directory