| Weight | 328.5 g/mol |
|---|---|
| Formula | C21H32N2O |
| Hydrogen Acceptors | 2 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 1 |
| Rotatable Bonds | 0 |
stanozolol (10418-03-8, 302-96-5)
Winstrol · Stanazolol · Stromba
 Score:
#494 in Biochemistry
,
#1565 in Biology
,
#1835 in Chemistry
Score:
#494 in Biochemistry
,
#1565 in Biology
,
#1835 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - stanozolol81.40 - 448.35 USD
- Fisher Scientific - Search for stanozolol
- TCI - Search for stanozolol
- Contrasting Effects of Testosterone and Stanozolol on Serum Lipoprotein Levels (JAMA, 1989)
- Reduction of lecithin-cholesterol acyltransferase, apolipoprotein D and the Lp(a) lipoprotein with the anabolic steroid stanozolol (Biochimica et Biophysica Acta, 1984)
- Stanozolol in postmenopausal osteoporosis: Therapeutic efficacy and possible mechanisms of action (Metabolism-clinical and Experimental, 1983)
- Reduction in high density lipoproteins by anabolic steroid (stanozolol) therapy for postmenopausal osteoporosis. (Metabolism-clinical and Experimental, 1982)
- Long-term detection and identification of metandienone and stanozolol abuse in athletes by gas chromatography-high-resolution mass spectrometry. (Journal of Chromatography B: Biomedical Sciences and Applications, 1996)
- Clinical and biochemical effects of stanozolol therapy for hereditary angioedema (The Journal of Allergy and Clinical Immunology, 1981)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Winstrol
- Stanazolol
- Stromba
- Zambon Brand of Stanozolol
- Sanofi Winthrop Brand of Stanozolol
- Methylstanazol
- Androstanazol
- Sanofi-Synthelabo Brand of Stanozolol
-
SMILESCC12CCC3C(C1CCC2(C)O)CCC4C3(CC5=C(C4)NN=C5)C
-
InChIKeyLKAJKIOFIWVMDJ-UHFFFAOYSA-N
- Pubchem - stanozolol
- Wikipedia - Stanozolol
Stanozolol, sold under many brand names, is an androgen and anabolic steroid (AAS) medication which was derived from dihydrotestosterone (DHT). It was developed by American pharmaceutical company Winthrop Laboratories (Sterling Drug) in 1962, and has been approved by the Food and Drug Administration for human use, though it is no longer marketed in this country. It is also used in veterinary medicine.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
21-Hydroxypregnenolone
(1164-98-3)
9 alternate names
3 suppliers
53-00-9
(53-00-9)
9 alternate names
3 suppliers
Methandienone
(72-63-9, 100-61-8)
9 alternate names
3 suppliers
norethynodrel
(68-23-5)
9 alternate names
3 suppliers
16alpha-Hydroxyestrone
(566-76-7)
9 alternate names
3 suppliers
2-HYDROXYESTRONE
(362-06-1)
9 alternate names
3 suppliers
estradiol cypionate
(313-06-4)
9 alternate names
3 suppliers
2-METHOXYESTRONE
(362-08-3)
9 alternate names
3 suppliers
302-97-6
(302-97-6)
9 alternate names
2 safety hazards
3 suppliers
desoxycorticosterone
(64-85-7)
9 alternate names
3 suppliers
TESTOSTERONE CYPIONATE
(58-20-8)
9 alternate names
3 suppliers
19-Hydroxyandrost-4-ene-3,17-dione
(510-64-5)
9 alternate names
3 suppliers
Tetrahydrodeoxycorticosterone
(567-03-3, 567-02-2)
9 alternate names
3 suppliers
Dihydrocholesterol
(80-97-7)
9 alternate names
3 suppliers
Spiroxamine
(118134-30-8)
9 alternate names
4 safety hazards
3 suppliers
IBOGAINE
(83-74-9)
9 alternate names
3 suppliers
Endabuse
(83-74-9)
9 alternate names
3 suppliers
IBOGAINE
(83-74-9)
9 alternate names
3 suppliers
ISOPHYTOL
(505-32-8)
9 alternate names
2 safety hazards
3 suppliers
27-hydroxycholesterol
(20380-11-4)
9 alternate names
3 suppliers
Lathosterol
(80-99-9)
9 alternate names
3 suppliers
Testosterone sulfate
(651-45-6)
9 alternate names
3 suppliers
Zymostenol
(566-97-2)
9 alternate names
3 suppliers
CAS Directory

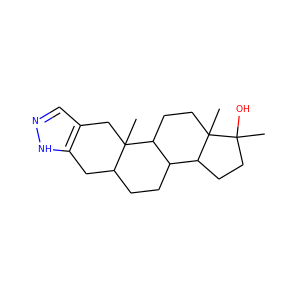
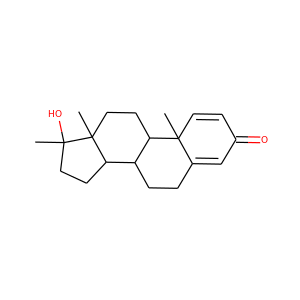

 stanozolol
stanozolol
