| Weight | 286.371 g/mol |
|---|---|
| Formula | C18H22O3 |
| Hydrogen Acceptors | 3 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 1 |
| Rotatable Bonds | 0 |
16alpha-Hydroxyestrone (566-76-7)
"What do you need help with?"
 LabBot
LabBot
- Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. (Environmental Health Perspectives, 1997)
- Predictors of the plasma ratio of 2-hydroxyestrone to 16alpha-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. (Carcinogenesis, 2003)
- Regulation of cell cycle and cyclins by 16alpha-hydroxyestrone in MCF-7 breast cancer cells (Journal of Molecular Endocrinology, 2001)
- Induction of 16alpha-/2-hydroxyestrone metabolite ratios in MCF-7 cells by pesticides, carcinogens, and antiestrogens does not predict mammary carcinogens. (Environmental Health Perspectives, 1998)
- Tissue-selective effects of continuous release of 2-hydroxyestrone and 16alpha-hydroxyestrone on bone, uterus and mammary gland in ovariectomized growing rats. (Journal of Endocrinology, 2001)
- [Physiological and therapeutic action of 16alpha-hydroxyestrone]. (Therapie, 1961)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC12CCC3C(C1CC(C2=O)O)CCC4=C3C=CC(=C4)O
-
InChIKeyWPOCIZJTELRQMF-UHFFFAOYSA-N
- Pubchem - 16alpha-Hydroxyestrone
- Wikipedia - 16-hydroxyestrone
16-Hydroxyestrone (16-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-trien-3,16-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16-hydroxyestrone may help to protect against osteoporosis.
Suppliers
Most-cited Publications
Safety
Alternate Names
External Links
Similar Compounds
norethynodrel
(68-23-5)
1 alternate name
3 suppliers
2-HYDROXYESTRONE
(362-06-1)
1 alternate name
3 suppliers
2-METHOXYESTRONE
(362-08-3)
1 alternate name
3 suppliers
19-Hydroxyandrost-4-ene-3,17-dione
(510-64-5)
1 alternate name
3 suppliers
21-Hydroxypregnenolone
(1164-98-3)
1 alternate name
3 suppliers
Methandienone
(72-63-9, 100-61-8)
1 alternate name
3 suppliers
302-97-6
(302-97-6)
1 alternate name
2 safety hazards
3 suppliers
53-00-9
(53-00-9)
1 alternate name
3 suppliers
desoxycorticosterone
(64-85-7)
1 alternate name
3 suppliers
stanozolol
(10418-03-8, 302-96-5)
1 alternate name
3 suppliers
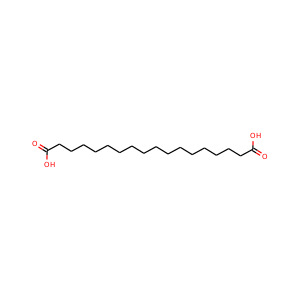
Octadecanedioic acid
(871-70-5)
1 alternate name
3 suppliers
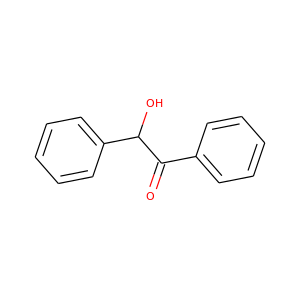
BENZOIN
(119-53-9, 8050-35-9, 9000-72-0, 9000-05-9, 579-44-2)
1 alternate name
3 suppliers
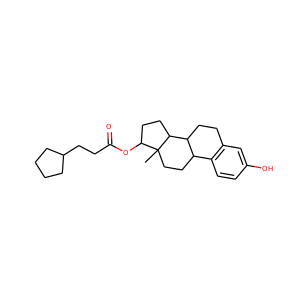
estradiol cypionate
(313-06-4)
1 alternate name
3 suppliers
L-thyronine
(1596-67-4)
1 alternate name
3 suppliers
SIDURON
(1982-49-6)
1 alternate name
3 suppliers
4376-20-9
(4376-20-9)
1 alternate name
3 suppliers
COUMESTROL
(479-13-0)
1 alternate name
3 suppliers
formononetin
(485-72-3)
1 alternate name
3 suppliers
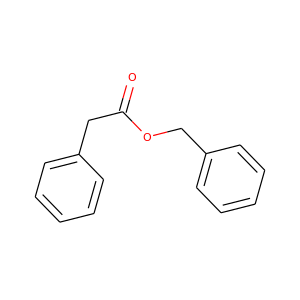
Benzyl phenylacetate
(102-16-9)
1 alternate name
3 suppliers
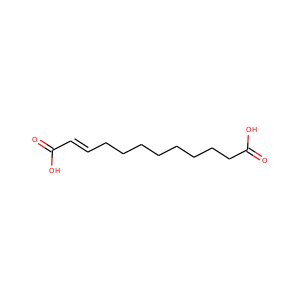
Traumatic acid
(6402-36-4, 124-00-5)
1 alternate name
3 suppliers
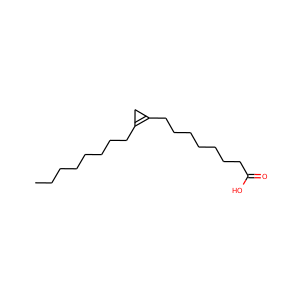
STERCULIC ACID
(55088-60-3, 738-87-4)
1 alternate name
3 suppliers
fluprednisolone
(53-34-9)
1 alternate name
3 suppliers
Pipradrol
(467-60-7)
1 alternate name
3 suppliers
CAS Directory

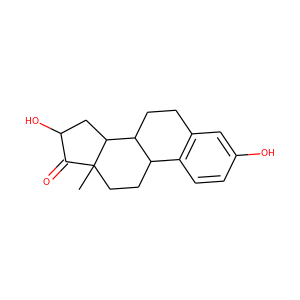
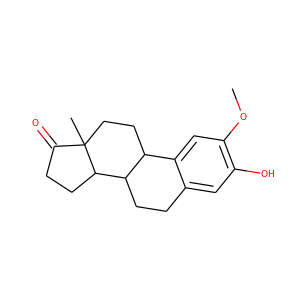
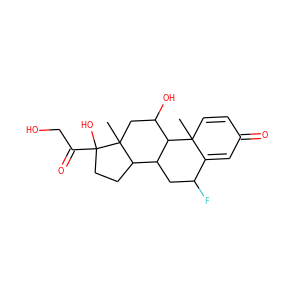

 16alpha-Hydroxyestrone
16alpha-Hydroxyestrone
