| Weight | 286.371 g/mol |
|---|---|
| Formula | C18H22O3 |
| Hydrogen Acceptors | 3 |
| Hydrogen Donors | 2 |
| Aromatic Rings | 1 |
| Rotatable Bonds | 0 |
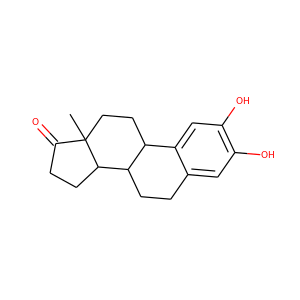
2-HYDROXYESTRONE (362-06-1)
"What do you need help with?"
 LabBot
LabBot
- Estrogen metabolism and risk of breast cancer: A prospective study of the 2:16-hydroxyestrone ratio in premenopausal and postmenopausal women (Epidemiology, 2000)
- Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. (Journal of Biological Chemistry, 1984)
- Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. (Environmental Health Perspectives, 1995)
- 2-Hydroxyestrone: the 'good' estrogen (Journal of Endocrinology, 1996)
- Urinary 2-Hydroxyestrone/16-Hydroxyestrone Ratio and Risk of Breast Cancer in Postmenopausal Women (Journal of the National Cancer Institute, 1999)
- Increased urinary excretion of 2-hydroxyestrone but not 16-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. (Cancer Research, 2000)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC12CCC3C(C1CCC2=O)CCC4=CC(=C(C=C34)O)O
-
InChIKeySWINWPBPEKHUOD-UHFFFAOYSA-N
- Pubchem - 2-HYDROXYESTRONE
- Wikipedia - 2-hydroxyestrone
2-Hydroxyestrone (2-OHE1), also known as estra-1,3,5(10)-trien-2,3-diol-17-one, is an endogenous, naturally occurring catechol estrogen and a major metabolite of estrone and estradiol. It is formed irreversibly from estrone in the liver and to a lesser extent in other tissues via 2-hydroxylation mediated by cytochrome P450 enzymes, mainly the CYP3A and CYP1A subfamilies. 2-OHE1 is the most abundant catechol estrogen in the body.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
2-METHOXYESTRONE
(362-08-3)
1 alternate name
3 suppliers
19-Hydroxyandrost-4-ene-3,17-dione
(510-64-5)
1 alternate name
3 suppliers
Methandienone
(72-63-9, 100-61-8)
1 alternate name
3 suppliers
16alpha-Hydroxyestrone
(566-76-7)
1 alternate name
3 suppliers
53-00-9
(53-00-9)
1 alternate name
3 suppliers
302-97-6
(302-97-6)
1 alternate name
2 safety hazards
3 suppliers
desoxycorticosterone
(64-85-7)
1 alternate name
3 suppliers
NONIVAMIDE
(2444-46-4)
1 alternate name
3 suppliers
Dihydrocapsaicin
(19408-84-5)
1 alternate name
3 suppliers
21-Hydroxypregnenolone
(1164-98-3)
1 alternate name
3 suppliers
Octyl gallate
(1034-01-1)
1 alternate name
1 safety hazard
3 suppliers
Dodecyl gallate
(1166-52-5)
1 alternate name
1 safety hazard
3 suppliers
norethynodrel
(68-23-5)
1 alternate name
3 suppliers
stanozolol
(10418-03-8, 302-96-5)
1 alternate name
3 suppliers
fluprednisolone
(53-34-9)
1 alternate name
3 suppliers
20-beta-Progerol
(145-15-3, 145-14-2)
1 alternate name
3 suppliers
2,4-DIHYDROXYBENZOPHENONE
(131-56-6)
1 alternate name
3 suppliers
RACTOPAMINE
(97825-25-7)
1 alternate name
3 suppliers
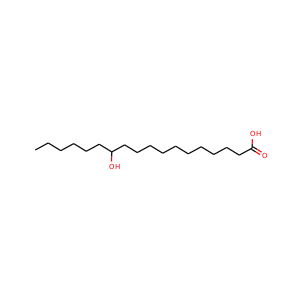
12-Hydroxystearic acid
(106-14-9, 36377-33-0, 18417-00-0, 27924-99-8, 5762-36-7)
1 alternate name
3 suppliers
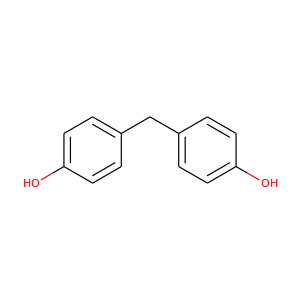
4,4'-Methylenediphenol
(620-92-8)
1 alternate name
3 suppliers
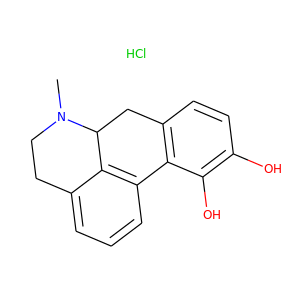
Apomorphine HCl
(314-19-2, 41372-20-7, 6208-08-8)
1 alternate name
3 suppliers
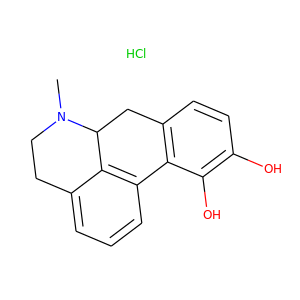
314-19-2
(314-19-2, 41372-20-7)
1 alternate name
3 suppliers
Piperalin
(3478-94-2)
1 alternate name
3 suppliers
Dextromethorphan hydrobromide
(125-69-9)
1 alternate name
3 suppliers
CAS Directory

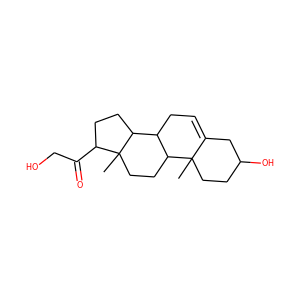

 2-HYDROXYESTRONE
2-HYDROXYESTRONE
