| Weight | 394.423 g/mol |
|---|---|
| Formula | C23H22O6 |
| Hydrogen Acceptors | 6 |
| Hydrogen Donors | 0 |
| Aromatic Rings | 2 |
| Rotatable Bonds | 3 |
rotenone (83-79-4)
 Score:
#25 in Biochemistry
,
#30 in Neuroscience
,
#165 in Biology
,
#170 in Chemistry
Score:
#25 in Biochemistry
,
#30 in Neuroscience
,
#165 in Biology
,
#170 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - rotenone19.10 - 527.00 USD
- Fisher Scientific - Search for rotenone
- TCI - rotenone112.00 - 391.00 USD
- Mitochondrial Complex I Inhibitor Rotenone Induces Apoptosis through Enhancing Mitochondrial Reactive Oxygen Species Production (Journal of Biological Chemistry, 2003)
- Mechanism of Toxicity in Rotenone Models of Parkinson's Disease (The Journal of Neuroscience, 2003)
- Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and -synuclein aggregation (Experimental Neurology, 2003)
- Rotenone, Paraquat, and Parkinsons Disease (Environmental Health Perspectives, 2011)
- Distinct Role for Microglia in Rotenone-Induced Degeneration of Dopaminergic Neurons (The Journal of Neuroscience, 2002)
- Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats (Behavioural Brain Research, 2002)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - H300: Fatal if swallowed
Danger Acute toxicity, oral - Category 1, 2 - H301: Toxic if swallowed
Danger Acute toxicity, oral - Category 3 - H315: Causes skin irritation
Warning Skin corrosion/irritation - Category 2 - H319: Causes serious eye irritation
Warning Serious eye damage/eye irritation - Category 2A - H370: Causes damage to organs
Danger Specific target organ toxicity, single exposure - Category 1 - H400: Very toxic to aquatic life
Warning Hazardous to the aquatic environment, acute hazard - Category 1 - H410: Very toxic to aquatic life with long lasting effects
Warning Hazardous to the aquatic environment, long-term hazard - Category 1 -
SMILESCC(=C)C1CC2=C(O1)C=CC3=C2OC4COC5=CC(=C(C=C5C4C3=O)OC)OC
-
InChIKeyJUVIOZPCNVVQFO-UHFFFAOYSA-N
- Pubchem - rotenone
- Wikipedia - rotenone
Rotenone is an odorless, colorless, crystalline isoflavone used as a broad-spectrum insecticide, piscicide, and pesticide. It occurs naturally in the seeds and stems of several plants, such as the jicama vine plant, and the roots of several members of Fabaceae. It was the first described member of the family of chemical compounds known as rotenoids.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
Xanthohumol
(6754-58-1)
1 alternate name
3 suppliers
Dibenz[a,h]acridine
(226-36-8)
1 alternate name
1 safety hazard
3 suppliers
hydroxyprogesterone
(604-09-1, 68-96-2)
1 alternate name
3 suppliers
EMETINE
(316-42-7, 483-18-1)
1 alternate name
3 suppliers
Oripavine
(467-04-9)
1 alternate name
3 suppliers
20-beta-Progerol
(145-15-3, 145-14-2)
1 alternate name
3 suppliers
17alpha-Hydroxypregn-4-ene-3,20-dione
(604-09-1)
1 alternate name
3 suppliers
1,1'-Bi-2-naphthol
(18531-99-2, 602-09-5, 18531-94-7)
1 alternate name
3 suppliers
21-Deoxyhydrocortisone
(641-77-0)
1 alternate name
3 suppliers
Benzo[j]fluoranthene
(205-82-3)
1 alternate name
3 safety hazards
3 suppliers
Apomorphine HCl
(314-19-2, 41372-20-7, 6208-08-8)
1 alternate name
3 suppliers
NAPTALAM
(132-66-1)
1 alternate name
3 suppliers
314-19-2
(314-19-2, 41372-20-7)
1 alternate name
3 suppliers
Melengestro acetate
(425-51-4, 595-33-5)
1 alternate name
3 suppliers
Lathosterol
(80-99-9)
1 alternate name
3 suppliers
7-Dehydrocholesterol
(434-16-2)
1 alternate name
3 suppliers
BENZO(G)CHRYSENE
(196-78-1)
1 alternate name
3 suppliers
Dihydrocapsaicin
(19408-84-5)
1 alternate name
3 suppliers
Benzo[c]chrysene
(194-69-4)
1 alternate name
3 suppliers
2-METHOXYESTRONE
(362-08-3)
1 alternate name
3 suppliers
Dibenz[a,h]anthracene
(53-70-3)
1 alternate name
4 safety hazards
3 suppliers
CHRYSENE
(65996-93-2, 50-32-8, 218-01-9)
1 alternate name
3 safety hazards
3 suppliers
3-methylcholanthrene
(56-49-5)
1 alternate name
3 suppliers
Benz[a]anthracene
(56-55-3)
1 alternate name
4 safety hazards
3 suppliers
CAS Directory

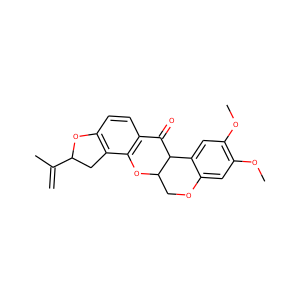

 rotenone
rotenone
