| Weight | 390.52 g/mol |
|---|---|
| Formula | C23H34O5 |
| Hydrogen Acceptors | 5 |
| Hydrogen Donors | 1 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 6 |
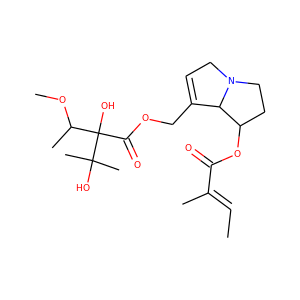
Mevastatin (73573-88-3)
 Score:
#695 in Neuroscience
,
#1252 in Biochemistry
,
#3095 in Biology
,
#3868 in Chemistry
Score:
#695 in Neuroscience
,
#1252 in Biochemistry
,
#3095 in Biology
,
#3868 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Mevastatin109.50 USD
- Fisher Scientific - Search for Mevastatin
- TCI - Mevastatin130.00 - 440.00 USD
- Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice (Stroke, 2001)
- Relative Lipophilicities, Solubilities, and StructurePharmacological Considerations of 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) Reductase Inhibitors Pravastatin, Lovastatin, Mevastatin, and Simvastatin (Journal of Pharmaceutical Sciences, 1991)
- HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2 (Carcinogenesis, 2001)
- Structural insights into drug processing by human carboxylesterase 1: tamoxifen, mevastatin, and inhibition by benzil. (Journal of Molecular Biology, 2005)
- Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. (Fertility and Sterility, 2004)
- The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells (BMC Cancer, 2008)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCCC(C)C(=O)OC1CCC=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O
-
InChIKeyAJLFOPYRIVGYMJ-UHFFFAOYSA-N
- Pubchem - Mevastatin
- Wikipedia - mevastatin
Mevastatin (compactin, ML-236B) is a hypolipidemic agent that belongs to the statins class. It was isolated from the mold Penicillium citrinum by Akira Endo in the 1970s, and he identified it as a HMG-CoA reductase inhibitor, i.e., a statin. Mevastatin might be considered the first statin drug; clinical trials on mevastatin were performed in the late 1970s in Japan, but it was never marketed.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
UNII-1R89KK61YI
(73573-88-3, 84064-38-0)
1 alternate name
3 suppliers
estradiol valerate
(979-32-8)
1 alternate name
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
1 alternate name
3 suppliers
Isolithocholic acid
(1534-35-6)
1 alternate name
3 suppliers
Enterolactone
(76543-15-2)
1 alternate name
3 suppliers
testosterone enanthate
(315-37-7)
1 alternate name
3 suppliers
ABIETIC ACID
(514-10-3)
1 alternate name
3 suppliers
Alitame
(80863-62-3)
1 alternate name
3 suppliers
CP 47497
(70434-82-1)
1 alternate name
3 suppliers
Nafenopin
(3771-19-5)
1 alternate name
3 suppliers
7-Ketodeoxycholic acid
(911-40-0)
1 alternate name
3 suppliers
Benzyl butyl phthalate
(85-68-7)
1 alternate name
4 safety hazards
3 suppliers
ERUCIC ACID
(1072-39-5, 112-86-7)
1 alternate name
3 suppliers
Methoprene
(36557-27-4, 40596-69-8, 52020-07-2)
1 alternate name
3 suppliers
LASIOCARPINE
(303-34-4)
1 alternate name
2 safety hazards
3 suppliers
CHLOROPROPYLATE
(5836-10-2)
1 alternate name
3 suppliers
DIETHYLCARBAMAZINE CITRATE
(1642-54-2)
1 alternate name
3 suppliers
Cerebrosterol
(474-73-7)
1 alternate name
3 suppliers
Lathosterol
(80-99-9)
1 alternate name
3 suppliers
PHYTANIC ACID
(14721-66-5)
1 alternate name
3 suppliers
CAS Directory

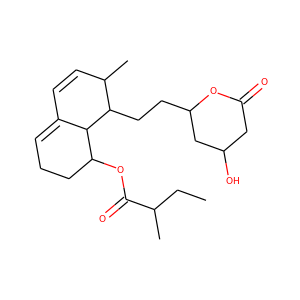
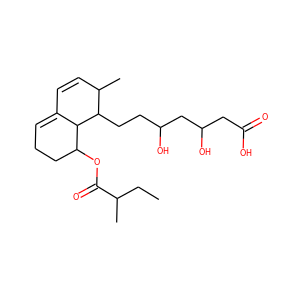
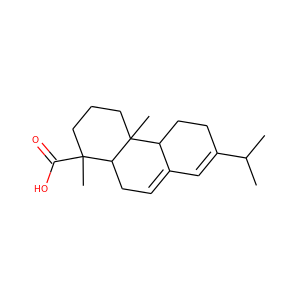
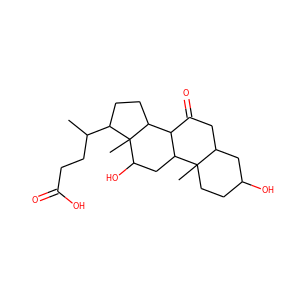

 Mevastatin
Mevastatin
