| Weight | 265.222 g/mol |
|---|---|
| Formula | C8H15N3O7 |
| Hydrogen Acceptors | 8 |
| Hydrogen Donors | 5 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 3 |
streptozocin (18883-66-4, 66395-18-4)
Streptozotocin · Zanosar · 2-Deoxy-2-((methylnitrosoamino)carbonyl)amino-D-glucose
 Score:
#224 in Neuroscience
,
#272 in Biochemistry
,
#1055 in Biology
,
#1177 in Chemistry
Score:
#224 in Neuroscience
,
#272 in Biochemistry
,
#1055 in Biology
,
#1177 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas (Physiological Research, 2001)
- The mechanisms of alloxan- and streptozotocin-induced diabetes. (Diabetologia, 2008)
- Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. (Diabetes, 2000)
- Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. (Pharmacological Research, 2005)
- Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus (Science, 1976)
- Diabetogenic action of streptozotocin: relationship of dose to metabolic response (Journal of Clinical Investigation, 1969)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - H301: Toxic if swallowed
Danger Acute toxicity, oral - Category 3 - H351: Suspected of causing cancer
Warning Carcinogenicity - Category 2 - H370: Causes damage to organs
Danger Specific target organ toxicity, single exposure - Category 1 - Streptozotocin
- Zanosar
- 2-Deoxy-2-((methylnitrosoamino)carbonyl)amino-D-glucose
- Streptozotocine
-
SMILESCN(C(=O)NC1C(C(C(OC1O)CO)O)O)N=O
-
InChIKeyZSJLQEPLLKMAKR-UHFFFAOYSA-N
- Pubchem - streptozocin
- Wikipedia - -streptozocin
Streptozotocin or streptozocin (INN, USP) (STZ) is a naturally occurring chemical that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Synthesis
Reactant
+
Methylnitrosamine
(Schotten-Baumann amide from acid)

Methylboronic acid
+
Reactant
(Chan-Lam coupling reaction)

Reactant
+
dimethyl sulfide
(Corey-Kim oxidation of secondary alcohols)

Similar Compounds
2-acetamido-2-deoxy-beta-D-glucopyranosylamine
(14131-68-1)
5 alternate names
3 suppliers
LINAMARIN
(554-35-8)
5 alternate names
3 suppliers
5,6-Dihydro-5-azacytidine
(62402-31-7, 62488-57-7)
5 alternate names
3 suppliers
d-iduronic acid
(3402-98-0)
5 alternate names
3 suppliers
Croscarmellose
(9000-11-7)
5 alternate names
3 suppliers
1,5-Anhydro-D-glucitol
(154-58-5)
5 alternate names
3 suppliers
l-Alanyl-l-glutamine
(39537-23-0)
5 alternate names
3 suppliers
D-manno-Heptulose
(29325-35-7, 3019-74-7, 3615-44-9)
5 alternate names
3 suppliers
N-Methyl-D-glucamine
(6284-40-8)
5 alternate names
3 suppliers
meglumine
(6284-40-8)
5 alternate names
3 suppliers
LINURON
(330-55-2)
5 alternate names
4 safety hazards
3 suppliers
MONOLINURON
(1746-81-2)
5 alternate names
3 safety hazards
3 suppliers
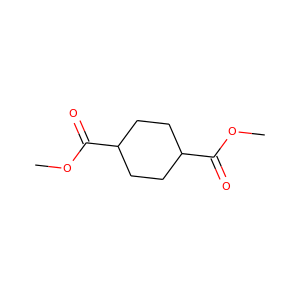
Dimethyl 1,4-cyclohexanedicarboxylate
(3399-22-2, 94-60-0, 3399-21-1)
5 alternate names
3 suppliers
DCNU
(69839-80-1)
5 alternate names
3 suppliers
Citric acid monohydrate
(5949-29-1)
5 alternate names
2 safety hazards
3 suppliers
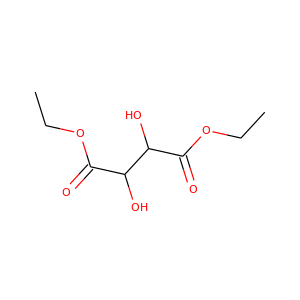
Diethyl tartrate
(87-91-2, 57968-71-5)
5 alternate names
3 suppliers
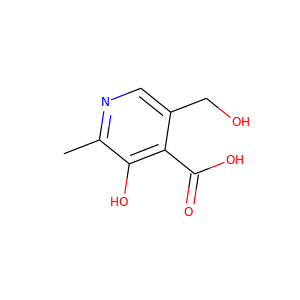
4-Pyridoxic acid
(82-82-6)
5 alternate names
3 suppliers
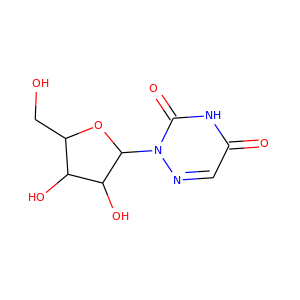
6-AZAURIDINE
(54-25-1)
5 alternate names
3 suppliers
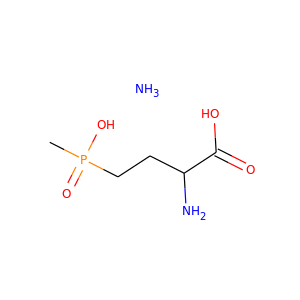
Glufosinate-ammonium
(77182-82-2)
5 alternate names
3 safety hazards
3 suppliers
METOBROMURON
(3060-89-7)
5 alternate names
3 suppliers
CHLORBROMURON
(13360-45-7)
5 alternate names
3 suppliers
Diammonium hydrogen citrate
(3012-65-5)
5 alternate names
3 suppliers
Citric acid, ammonium salt
(7632-50-0)
5 alternate names
3 suppliers
N-Acetyl-L-aspartic acid
(997-55-7)
5 alternate names
3 suppliers
1,6-Anhydro-beta-D-glucopyranose
(498-07-7)
5 alternate names
3 suppliers
D-Isoglucosamine
(4429-04-3)
5 alternate names
3 suppliers
Leucoglucosan
(498-07-7)
5 alternate names
3 suppliers
CAS Directory


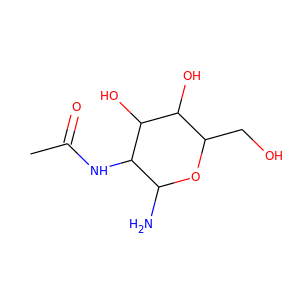
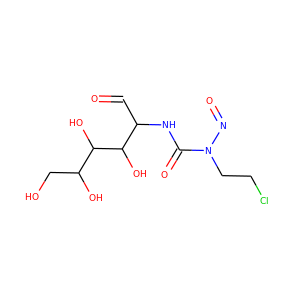

 streptozocin
streptozocin
