| Weight | 426.729 g/mol |
|---|---|
| Formula | C30H50O |
| Hydrogen Acceptors | 1 |
| Hydrogen Donors | 1 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 4 |
lanosterol (79-63-0)
Kryptosterol · 4,4,14 alpha-trimethyl-5 alpha-cholesta-8,24-dien-3 beta-ol
 Score:
#1712 in Biochemistry
,
#3806 in Biology
,
#4789 in Chemistry
Score:
#1712 in Biochemistry
,
#3806 in Biology
,
#4789 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - lanosterol92.40 - 316.50 USD
- Fisher Scientific - Search for lanosterol
- TCI - Search for lanosterol
- The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. (Antimicrobial Agents and Chemotherapy, 1997)
- Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol (Cell Metabolism, 2005)
- From Lanosterol to Cholesterol: Structural Evolution and Differential Effects on Lipid Bilayers (Biophysical Journal, 2002)
- Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: a comparative study using 2H-, 13C- and 31p-NMR spectroscopy (Biochimica et Biophysica Acta, 1995)
- Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen (Proceedings of the National Academy of Sciences of the United States of America, 1993)
- A Three-Dimensional Model of Lanosterol 14-Demethylase of Candida albicans and Its Interaction with Azole Antifungals (Journal of Medicinal Chemistry, 2000)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Kryptosterol
- 4,4,14 alpha-trimethyl-5 alpha-cholesta-8,24-dien-3 beta-ol
-
SMILESCC(CCC=C(C)C)C1CCC2(C1(CCC3=C2CCC4C3(CCC(C4(C)C)O)C)C)C
-
InChIKeyCAHGCLMLTWQZNJ-UHFFFAOYSA-N
- Pubchem - lanosterol
- Wikipedia - lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
4,4-Dimechol-8,14,24-trienol
(64284-64-6)
3 alternate names
3 suppliers
Zymosterol
(128-33-6)
3 alternate names
3 suppliers
DESMOSTEROL
(313-04-2)
3 alternate names
3 suppliers
STIGMASTEROL
(83-48-7)
3 alternate names
3 suppliers
27-hydroxycholesterol
(20380-11-4)
3 alternate names
3 suppliers
22R-hydroxycholesterol
(22348-64-7, 17954-98-2)
3 alternate names
3 suppliers
Zymostenol
(566-97-2)
3 alternate names
3 suppliers
BETA-SITOSTEROL
(68555-08-8, 19044-06-5, 83-46-5)
3 alternate names
3 suppliers
Cerebrosterol
(474-73-7)
3 alternate names
3 suppliers
Dihydrocholesterol
(80-97-7)
3 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
3 alternate names
3 suppliers
Lathosterol
(80-99-9)
3 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
3 alternate names
3 suppliers
7-Dehydrocholesterol
(434-16-2)
3 alternate names
3 suppliers
Coprocholic acid
(547-98-8)
3 alternate names
3 suppliers
Glycolithocholic acid
(474-74-8)
3 alternate names
3 suppliers
Glycocholic acid
(475-31-0)
3 alternate names
3 suppliers
17-Hydroxypregnenolone
(387-79-1)
3 alternate names
3 suppliers
3,7-Dihydroxy-12-oxocholanoic acid
(2458-08-4)
3 alternate names
3 suppliers
7-Ketodeoxycholic acid
(911-40-0)
3 alternate names
3 suppliers
7alpha-Hydroxy-4-cholesten-3-one
(3862-25-7)
3 alternate names
3 suppliers
4-Cholesten-3-one
(601-57-0)
3 alternate names
3 suppliers
testosterone enanthate
(315-37-7)
3 alternate names
3 suppliers
estradiol valerate
(979-32-8)
3 alternate names
3 suppliers
Norethindrone acetate
(38673-38-0, 51-98-9)
3 alternate names
3 suppliers
19-Norethindrone acetate
(51-98-9)
3 alternate names
3 suppliers
CAS Directory

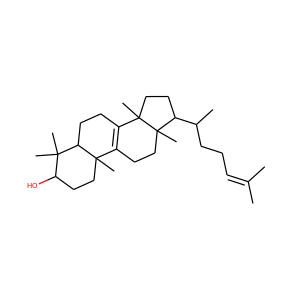
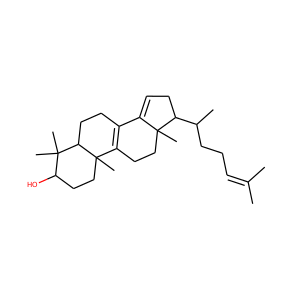
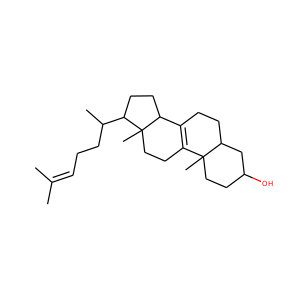
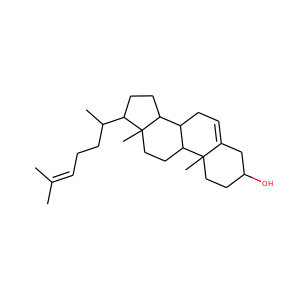
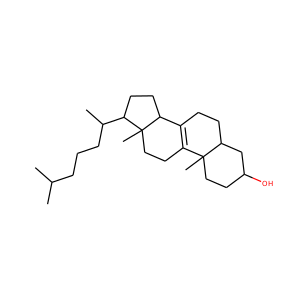
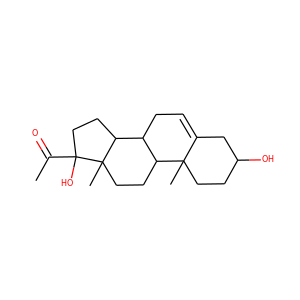
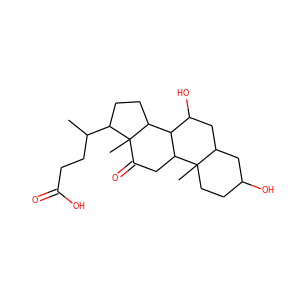
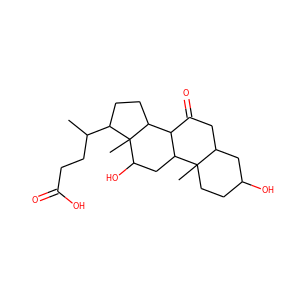
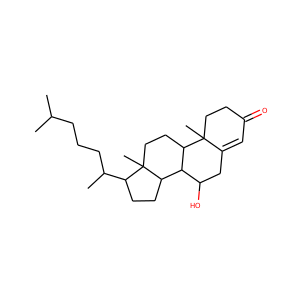
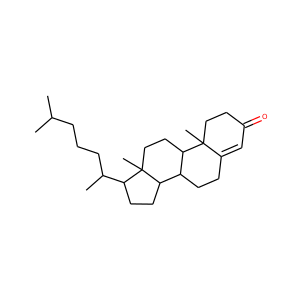

 lanosterol
lanosterol
