| Weight | 396.659 g/mol |
|---|---|
| Formula | C28H44O |
| Hydrogen Acceptors | 1 |
| Hydrogen Donors | 1 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 4 |
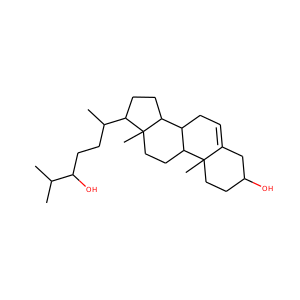
ERGOSTEROL (57-87-4)
Lumisterol · Provitamin D 2 · Pro Vitamin D2
 Score:
#157 in Microbiology
,
#536 in Biochemistry
,
#1638 in Biology
,
#1932 in Chemistry
Score:
#157 in Microbiology
,
#536 in Biochemistry
,
#1638 in Biology
,
#1932 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium (Journal of Cellular and Comparative Physiology, 1953)
- Ergosterol-to-Biomass Conversion Factors for Aquatic Hyphomycetes (Applied and Environmental Microbiology, 1993)
- Ergosterol and microbial biomass relationship in soil (Biology and Fertility of Soils, 1996)
- Ergosterol as a Measure of Fungal Growth (Phytopathology, 1979)
- ON THE STIMULATION OF NEW BONE-FORMATION WITH PARATHYROID EXTRACT AND IRRADIATED ERGOSTEROL (Endocrinology, 1932)
- Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. (Chemico-Biological Interactions, 1978)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Lumisterol
- Provitamin D 2
- Pro Vitamin D2
- Pro-Vitamin D2
-
SMILESCC(C)C(C)C=CC(C)C1CCC2C1(CCC3C2=CC=C4C3(CCC(C4)O)C)C
-
InChIKeyDNVPQKQSNYMLRS-UHFFFAOYSA-N
- Pubchem - ERGOSTEROL
- Wikipedia - ergosterol
Ergosterol (ergosta-5,7,22-trien-3-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that create it have become important targets for drug discovery. Ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.
Suppliers
Most-cited Publications
Areas of Application
Safety
Trends
Alternate Names
External Links
Similar Compounds
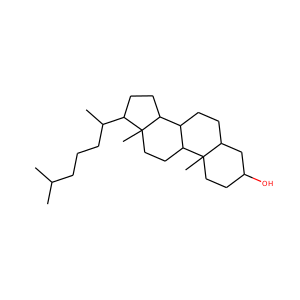
7-Dehydrocholesterol
(434-16-2)
5 alternate names
3 suppliers
Lathosterol
(80-99-9)
5 alternate names
3 suppliers
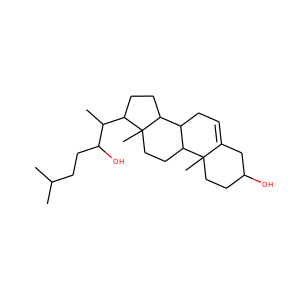
Cerebrosterol
(474-73-7)
5 alternate names
3 suppliers
Dihydrocholesterol
(80-97-7)
5 alternate names
3 suppliers
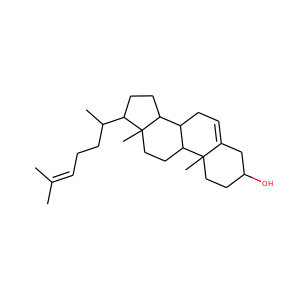
DESMOSTEROL
(313-04-2)
5 alternate names
3 suppliers
22R-hydroxycholesterol
(22348-64-7, 17954-98-2)
5 alternate names
3 suppliers
Zymostenol
(566-97-2)
5 alternate names
3 suppliers
Zymosterol
(128-33-6)
5 alternate names
3 suppliers
LITHOCHOLIC ACID
(434-13-9)
5 alternate names
3 suppliers
27-hydroxycholesterol
(20380-11-4)
5 alternate names
3 suppliers
Isolithocholic acid
(1534-35-6)
5 alternate names
3 suppliers
Glycolithocholic acid
(474-74-8)
5 alternate names
3 suppliers
STIGMASTEROL
(83-48-7)
5 alternate names
3 suppliers
BETA-SITOSTEROL
(68555-08-8, 19044-06-5, 83-46-5)
5 alternate names
3 suppliers
4,4-Dimechol-8,14,24-trienol
(64284-64-6)
5 alternate names
3 suppliers
17-Hydroxypregnenolone
(387-79-1)
5 alternate names
3 suppliers
Coprocholic acid
(547-98-8)
5 alternate names
3 suppliers
4-Cholesten-3-one
(601-57-0)
5 alternate names
3 suppliers
7-Ketodeoxycholic acid
(911-40-0)
5 alternate names
3 suppliers
7alpha-Hydroxy-4-cholesten-3-one
(3862-25-7)
5 alternate names
3 suppliers
Glycocholic acid
(475-31-0)
5 alternate names
3 suppliers
3,7-Dihydroxy-12-oxocholanoic acid
(2458-08-4)
5 alternate names
3 suppliers
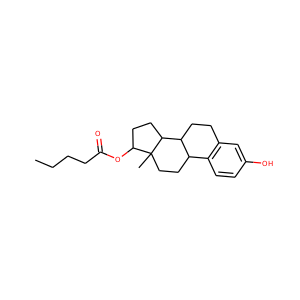
estradiol valerate
(979-32-8)
5 alternate names
3 suppliers
21-Hydroxypregnenolone
(1164-98-3)
5 alternate names
3 suppliers
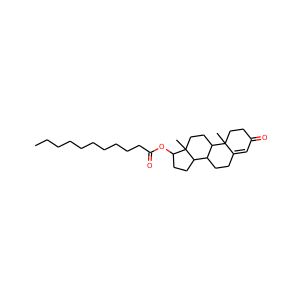
Testosterone undecanoate
(5949-44-0)
5 alternate names
3 suppliers
53-00-9
(53-00-9)
5 alternate names
3 suppliers
testosterone enanthate
(315-37-7)
5 alternate names
3 suppliers
CAS Directory

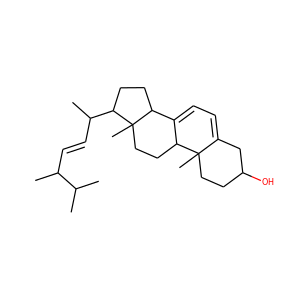
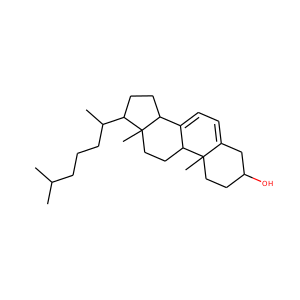
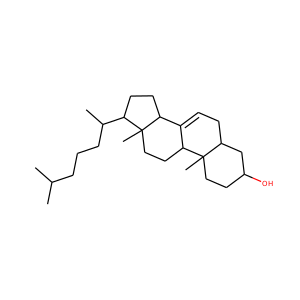
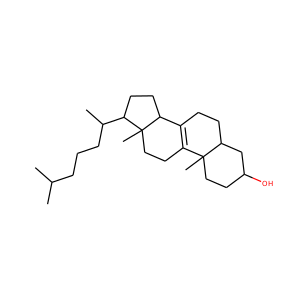
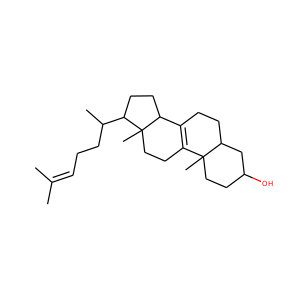
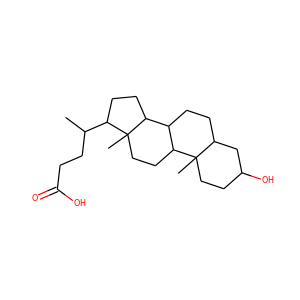
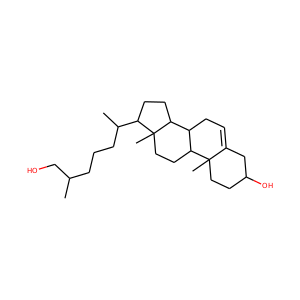
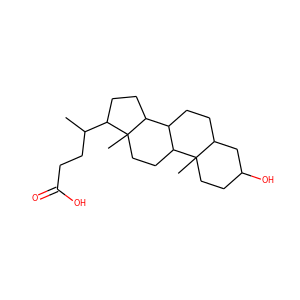
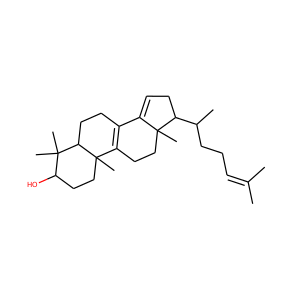

 ERGOSTEROL
ERGOSTEROL
