| Weight | 628.814 g/mol |
|---|---|
| Formula | C37H48N4O5 |
| Hydrogen Acceptors | 5 |
| Hydrogen Donors | 4 |
| Aromatic Rings | 3 |
| Rotatable Bonds | 15 |
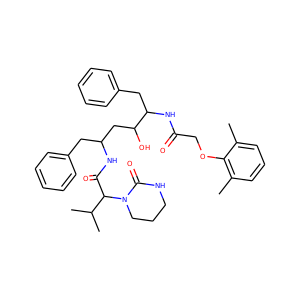
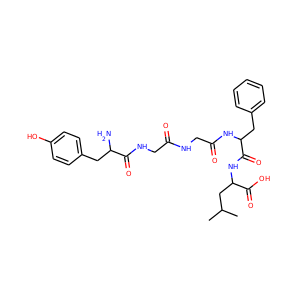
Lopinavir (192725-17-0)
 Score:
#1 in Virology
,
#33 in Microbiology
,
#554 in Biology
,
#589 in Chemistry
Score:
#1 in Virology
,
#33 in Microbiology
,
#554 in Biology
,
#589 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for Lopinavir
- Fisher Scientific - Search for Lopinavir
- TCI - Search for Lopinavir
- LopinavirRitonavir versus Nelfinavir for the Initial Treatment of HIV Infection (The New England Journal of Medicine, 2002)
- Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study (The Lancet, 2008)
- Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48 (AIDS, 2008)
- The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks : a randomised non-inferiority trial (The Lancet, 2006)
- Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial (The Lancet, 2007)
- Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. (AIDS, 2005)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. -
SMILESCC1=C(C(=CC=C1)C)OCC(=O)NC(CC2=CC=CC=C2)C(CC(CC3=CC=CC=C3)NC(=O)C(C(C)C)N4CCCNC4=O)O
-
InChIKeyKJHKTHWMRKYKJE-UHFFFAOYSA-N
- Pubchem - Lopinavir
- Wikipedia - lopinavir
Lopinavir (ABT-378) is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir, under the trade names Kaletra (high-income countries) and Aluvia (low-income countries). It was first approved by the FDA on 15 September 2000.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Synthesis
Reactant
+
CID 10139870
(Schotten-Baumann amide from acid)

Similar Compounds
Nelfinavir mesylate
(159989-65-8)
1 alternate name
3 suppliers
ONO-RS 411
(103177-37-3)
1 alternate name
3 suppliers
Bromadiolone
(28772-56-7)
1 alternate name
3 suppliers
UNII-X00B0D5O0E
(66258-76-2)
1 alternate name
3 suppliers
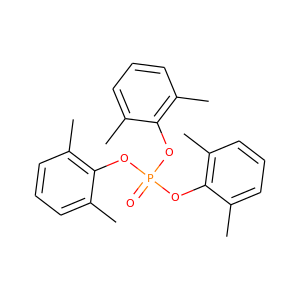
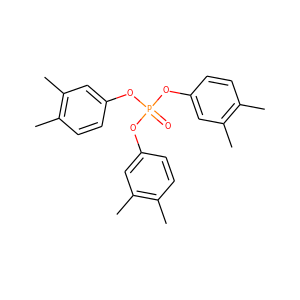
Tri-2,6-xylyl phosphate
(121-06-2)
1 alternate name
3 suppliers
19074-59-0
(19074-59-0)
1 alternate name
3 suppliers
Canthaxanthin
(514-78-3)
1 alternate name
3 suppliers
KADETHRIN
(58769-20-3)
1 alternate name
3 suppliers
KADETHRIN
(58769-20-3)
1 alternate name
3 suppliers
KADETHRIN
(58769-20-3)
1 alternate name
3 suppliers
KADETHRIN
(58769-20-3)
1 alternate name
3 suppliers
Capsanthin
(465-42-9)
1 alternate name
3 suppliers
NICARDIPINE HYDROCHLORIDE
(54527-84-3, 69441-18-5)
1 alternate name
3 suppliers
25653-16-1
(25653-16-1)
1 alternate name
3 suppliers
Meclizine dihydrochloride
(1104-22-9, 31884-77-2)
1 alternate name
3 suppliers
Clomifene citrate
(50-41-9, 7619-53-6)
1 alternate name
3 suppliers
Meclizine hydrochloride
(410538-37-3, 36236-67-6, 31884-77-2)
1 alternate name
3 suppliers
suxibuzone
(27470-51-5)
1 alternate name
3 suppliers
Totm
(3319-31-1, 82643-26-3)
1 alternate name
3 suppliers
Trixylenyl phosphate
(25155-23-1, 3862-12-2)
1 alternate name
3 suppliers
3319-31-1
(3319-31-1)
1 alternate name
3 suppliers
3319-31-1
(3319-31-1)
1 alternate name
3 suppliers
3319-31-1
(3319-31-1)
1 alternate name
3 suppliers
3319-31-1
(3319-31-1)
1 alternate name
3 suppliers
FENTANYL CITRATE
(990-73-8)
1 alternate name
3 suppliers
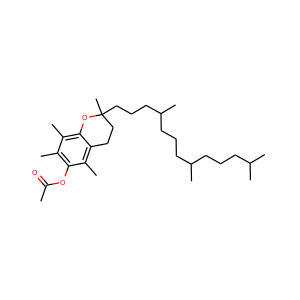
Vitamin E acetate
(7695-91-2, 52225-20-4, 58-95-7)
1 alternate name
3 suppliers
Tris(3,4-dimethylphenyl) phosphate
(3862-11-1)
1 alternate name
3 suppliers
Tolterodine tartrate
(124937-52-6)
1 alternate name
3 suppliers
Tamoxifen citrate
(54965-24-1)
1 alternate name
3 suppliers
Leu-enkephalin
(14-18-6, 58822-25-6, 59141-40-1)
1 alternate name
3 suppliers
Bazedoxifene acetate
(198481-33-3)
1 alternate name
3 suppliers
vitamin e succinate
(4345-03-3, 17407-37-3)
1 alternate name
3 suppliers
alpha-tochopheryl acetate
(7695-91-2, 58-95-7)
1 alternate name
3 suppliers
Trilinolenin
(14465-68-0)
1 alternate name
3 suppliers
PAMOIC ACID
(130-85-8)
1 alternate name
3 suppliers
CYHALOTHRIN
(68085-85-8, 91465-08-6)
1 alternate name
4 safety hazards
3 suppliers
Olio di ricino
(8001-79-4)
1 alternate name
3 suppliers
Flufenoxuron
(101463-69-8)
1 alternate name
2 safety hazards
3 suppliers
Tri-m-tolyl phosphate
(563-04-2)
1 alternate name
3 suppliers
Imatinib mesylate
(220127-57-1)
1 alternate name
3 suppliers
glimepiride
(684286-46-2, 93479-97-1)
1 alternate name
3 suppliers
Solifenacin succinate
(242478-38-2, 242478-37-1)
1 alternate name
3 suppliers
Tris(4-isopropylphenyl) phosphate
(2502-15-0, 68937-41-7, 26967-76-0)
1 alternate name
3 suppliers
Solvent green 3
(128-80-3)
1 alternate name
3 suppliers
Cyhalothrin solution
(68085-85-8, 91465-08-6)
1 alternate name
6 safety hazards
3 suppliers
Fluphenazine decanoate
(5002-47-1)
1 alternate name
3 suppliers
CAS Directory