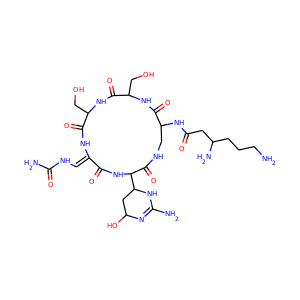
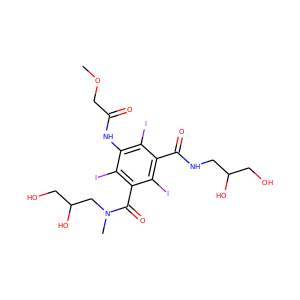
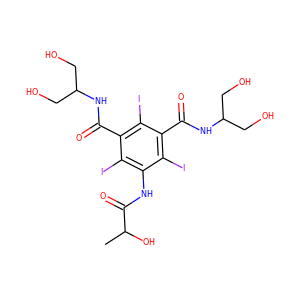
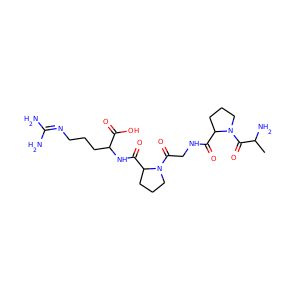
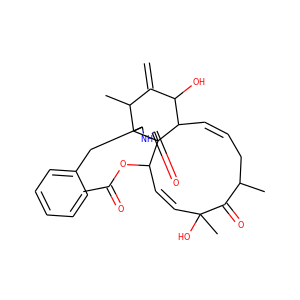
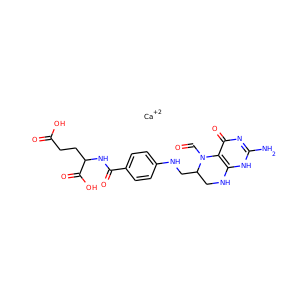
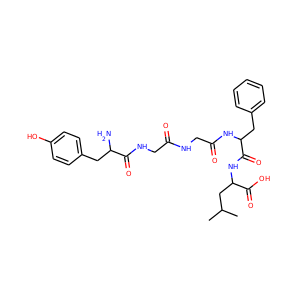
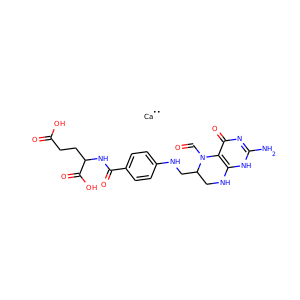
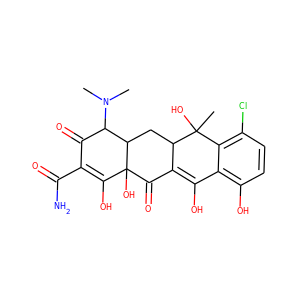
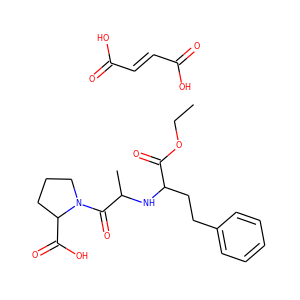
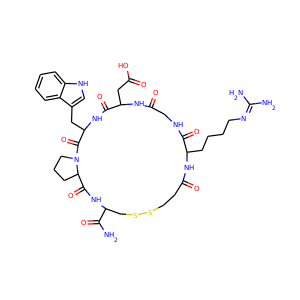
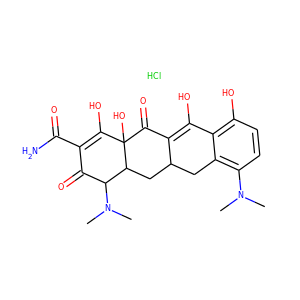
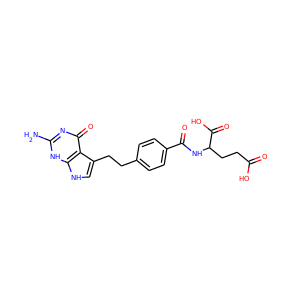
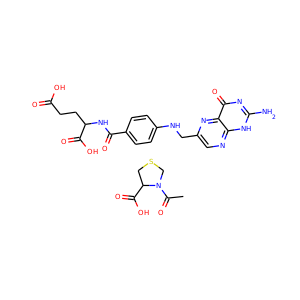
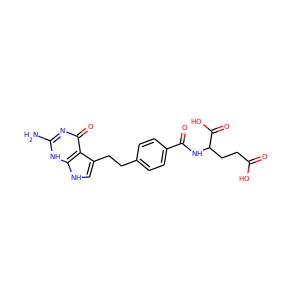
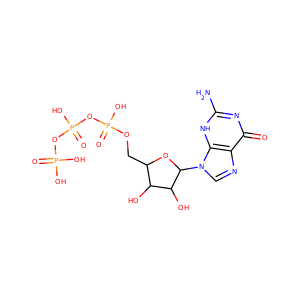
| Weight | 685.7 g/mol |
|---|---|
| Formula | C25H43N13O10 |
| Hydrogen Acceptors | 15 |
| Hydrogen Donors | 15 |
| Aromatic Rings | 0 |
| Rotatable Bonds | 10 |
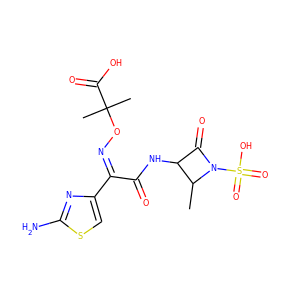
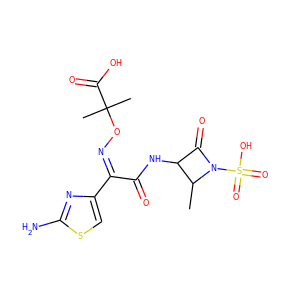
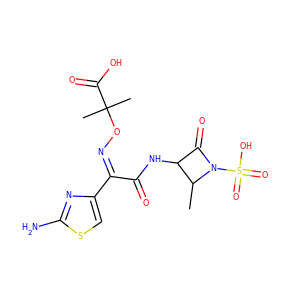
VIOMYCIN (32988-50-4, 37883-00-4)
Viocin · Vinactane · Viomicin
 Score:
#508 in Microbiology
,
#1881 in Biochemistry
,
#4036 in Biology
,
#5088 in Chemistry
Score:
#508 in Microbiology
,
#1881 in Biochemistry
,
#4036 in Biology
,
#5088 in Chemistry
"What do you need help with?"
 LabBot
LabBot
- Sigma-Aldrich - Search for VIOMYCIN
- Fisher Scientific - Search for VIOMYCIN
- TCI - Search for VIOMYCIN
- Molecular Analysis of Cross-Resistance to Capreomycin, Kanamycin, Amikacin, and Viomycin in Mycobacterium tuberculosis (Antimicrobial Agents and Chemotherapy, 2005)
- The antibiotic viomycin traps the ribosome in an intermediate state of translocation (Nature Structural & Molecular Biology, 2007)
- The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome (Nature Structural & Molecular Biology, 2010)
- The Inhibition of Ribosomal Translocation by Viomycin (FEBS Journal, 1977)
- The Allosteric Three-site Model for the Ribosomal Elongation Cycle NEW INSIGHTS INTO THE INHIBITION MECHANISMS OF AMINOGLYCOSIDES, THIOSTREPTON, AND VIOMYCIN* (Journal of Biological Chemistry, 1988)
- Molecular analysis of kanamycin and viomycin resistance in Mycobacterium smegmatis by use of the conjugation system. (Journal of Bacteriology, 1997)
-
Predict GHS Hazards for Any Chemical in silico.
Including Acute Oral Tox, Skin Sensitization, Eye Irritation, Aquatic Tox, & more. - Viocin
- Vinactane
- Viomicin
- Celiomycin
- Florimycin
- Tuberactinomycin B
- Viomycins
-
SMILESC1C(NC(=NC1O)N)C2C(=O)NCC(C(=O)NC(C(=O)NC(C(=O)NC(=CNC(=O)N)C(=O)N2)CO)CO)NC(=O)CC(CCCN)N
-
InChIKeyGXFAIFRPOKBQRV-UHFFFAOYSA-N
- Pubchem - VIOMYCIN
- Wikipedia - viomycin sulfate
Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis properties. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin.
Suppliers
Most-cited Publications
Areas of Application
Safety
Alternate Names
External Links
Similar Compounds
Chloropeptide
(12663-46-6)
8 alternate names
3 suppliers
Romidepsin
(128517-07-7)
8 alternate names
3 suppliers
iopromide
(73334-07-3)
8 alternate names
3 suppliers
iopamidol
(60166-93-0)
8 alternate names
3 suppliers
Antimycin A
(1397-94-0, 642-15-9, 116095-18-2)
8 alternate names
3 suppliers
antimycin A1
(1397-94-0, 642-15-9)
8 alternate names
3 suppliers
Enterostatin
(9001-78-9, 117830-79-2)
8 alternate names
3 suppliers
Deferoxamine mesylate
(138-14-7)
8 alternate names
3 suppliers
Leupeptin
(103476-89-7, 24365-47-7, 55123-66-5)
8 alternate names
3 suppliers
117467-28-4
(117467-28-4)
8 alternate names
3 suppliers
cefuroxime axetil
(64544-07-6)
8 alternate names
3 suppliers
glimepiride
(684286-46-2, 93479-97-1)
8 alternate names
3 suppliers
usnic acid
(125-46-2)
8 alternate names
3 suppliers
Deferoxamine hydrochloride
(1950-39-6)
8 alternate names
3 suppliers
Halosulfuron-methyl
(100784-20-1)
8 alternate names
1 safety hazard
3 suppliers
10-Fthf
(2800-34-2)
8 alternate names
3 suppliers
Tebufenozide
(112410-23-8, 142583-69-5)
8 alternate names
4 safety hazards
3 suppliers
aztreonam
(78110-38-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
Calcium leucovorin
(1492-18-8)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
cytochalasin D
(22144-77-0)
8 alternate names
3 suppliers
Leu-enkephalin
(14-18-6, 58822-25-6, 59141-40-1)
8 alternate names
3 suppliers
1492-18-8
(1492-18-8)
8 alternate names
3 suppliers
chlortetracycline
(57-62-5)
8 alternate names
3 suppliers
Azthreonam
(85506-30-5, 78110-38-0)
8 alternate names
3 suppliers
UNII-5Q55M3D9UA
(99341-02-3, 78110-38-0)
8 alternate names
3 suppliers
SAICAR
(3031-95-6)
8 alternate names
3 suppliers
DSSTox_CID_2640
(78110-38-0)
8 alternate names
3 suppliers
85506-30-5
(85506-30-5, 78110-38-0)
8 alternate names
3 suppliers
doxycycline
(564-25-0)
8 alternate names
3 suppliers
Enalapril maleate
(75847-73-3, 76095-16-4, 76420-75-2)
8 alternate names
3 suppliers
Eptifibatide
(188627-80-7)
8 alternate names
3 suppliers
MINOCYCLINE HYDROCHLORIDE
(13614-98-7)
8 alternate names
3 suppliers
Alimta
(150399-23-8)
8 alternate names
3 suppliers
Folcysteine
(8064-47-9)
8 alternate names
3 suppliers
Totm
(3319-31-1, 82643-26-3)
8 alternate names
3 suppliers
Pemetrexed
(137281-23-3)
8 alternate names
3 suppliers
3319-31-1
(3319-31-1)
8 alternate names
3 suppliers
GUANOSINE TRIPHOSPHATE
(36051-31-7, 86-01-1, 56001-37-7)
8 alternate names
3 suppliers
3319-31-1
(3319-31-1)
8 alternate names
3 suppliers
3319-31-1
(3319-31-1)
8 alternate names
3 suppliers
3319-31-1
(3319-31-1)
8 alternate names
3 suppliers
hyperforin
(11079-53-1)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
Clindamycin phosphate
(24729-96-2)
8 alternate names
3 suppliers
CAS Directory